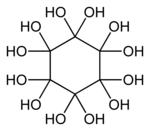
Dodecahydroxycyclohexane
| |

| |
| Names | |
|---|---|
| IUPAC name
cyclohexane-1,1,2,2,3,3,4,4,5,5,6,6-dodecol
| |
| Other names
dodecahydroxycyclohexane
| |
| Identifiers | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C6O12H12 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone (C6O6).
Dihydrate
The dihydrate C6O12H12·2H2O can be crystallized from methanol as colorless plates or prisms, that decompose at about 100 C. [1]

This compound was synthetized by J. Lerch[2] in 1862 by oxidation of hexahydroxybenzene C6(OH)6 or tetrahydroxy-p-benzoquinone C6(OH)4O2 and characterized by R. Nietzki and others in 1885 [3], although the product was for a long time assumed to be hexaketocyclohexane with water of crystallization (C6O6·8H2O).
Indeed, this product is still commonly marketed as cyclohexanehexone octahydrate, hexaketocyclohexane octahydrate, triquinoyl octahydrate and similar names. Its true nature was suspected since the 1950s or earlier [4], but was confirmed by X-ray diffraction analysis only in 2005[5]
See also
References
- ^
Alexander J. Fatiadi (1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)" (PDF). Journal of Research of the National Bureau of Standards A: Physics and Chemistry. 67A (2): 153–162.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Jos. Ud. Lerch (1862). "Ueber Kohlenoxydkalium und die aus demselben darstellbaren Säuren". Journal für Praktische Chemie. 87 (1): 427–469. doi:10.1002/prac.18620870146.
- ^ R. Nietzki, Th. Benckiser (1885). "Ueber Hexaoxybenzolderivate und ihre Beziehungen zur Krokonsäure und Rhodizonsäure". Berichte der deutschen chemischen Gesellschaft. 18 (1): 499–515. doi:10.1002/cber.188501801110.
- ^ Willis B. Person and Dale G. Williams (1957). "Infrared spectra and the structures of leuconic acid and triquinoyl". J. Phys. Chem. 61 (7): 1017–1018. doi:10.1021/j150553a047.
- ^
Thomas M. Klapötke (2005). "Dodecahydroxycyclohexane dihydrate" (PDF). Acta Crystallographica, Section E - Structure Reports Online.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
