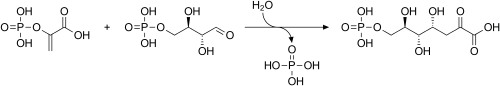Erythrose 4-phosphate
Appearance

| |

| |
| Names | |
|---|---|
| Other names
E4P
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | erythrose+4-phosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H9O7P | |
| Molar mass | 200.084 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Erythrose 4-phosphate is a phosphate of the simple sugar erythrose. It is an intermediate in the pentose phosphate pathway and the Calvin cycle.[1]
In addition, it serves as a precursor in the biosynthesis of the aromatic amino acids tyrosine, phenylalanine, and tryptophan. It is used in the first step of the shikimate pathway. At this stage, phosphoenolpyruvate and erythrose-4-phosphate react to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase.
It also used in 3-hydroxy-1-aminoacetone phosphate biosynthesis, which is a precursor of vitamin B6 in DXP-dependent pathway. Erythrose-4-phosphate dehydrogenase is used to produce erythronate-4-phosphate.
References
- ^ Schramm, M.; Racker, E. (1957). "Formation of Erythrose-4-phosphate and Acetyl Phosphate by a Phosphorolytic Cleavage of Fructose-6-phosphate". Nature. 179 (4574): 1349–1350. Bibcode:1957Natur.179.1349S. doi:10.1038/1791349a0. PMID 13451617. S2CID 1541286.