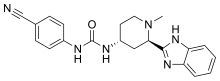
Glasdegib
 | |
| Clinical data | |
|---|---|
| Other names | PF-04449913 |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.244.738 |
| Chemical and physical data | |
| Formula | C21H22N6O |
| Molar mass | 374.448 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Glasdegib (PF-04449913) is an experimental cancer drug developed by Pfizer. It is a small molecule inhibitor of the Sonic hedgehog pathway, which is overexpressed in many types of cancer. It inhibits smoothened receptor, as do most drug in its class.[2]
Four phase II clinical trials are in progress. One is evaluating the efficacy of glasdegib in treating myelofibrosis in patients who were unable to control the disease with ruxolitinib.[3] Another is a combination trial of glasdenib with ARA-C, decitabine, daunorubicin, or cytarabine for the treatment of acute myeloid leukemia.[4] The third is for the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia.[5] The fourth administers glasdegib to patients at high risk for relapse after stem cell transplants in acute lymphoblastic or myelogenous leukemia.[6]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Glasdegib - AdisInsight". Adisinsight.springer.com. Retrieved 2017-05-22.
- ^ "Single-Agent Glasdegib In Patients With Myelofibrosis Previously Treated With Ruxolitinib - Full Text View". ClinicalTrials.gov. Retrieved 2017-05-22.
- ^ "A Study To Evaluate PF-04449913 With Chemotherapy In Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome - Full Text View". ClinicalTrials.gov. Retrieved 2017-05-22.
- ^ "Phase II Hedgehog Inhibitor for Myelodysplastic Syndrome (MDS) - Full Text View". ClinicalTrials.gov. Retrieved 2017-05-22.
- ^ "PF-04449913 For Patients With Acute Myeloid Leukemia at High Risk of Relapse After Donor Stem Cell Transplant - Full Text View". ClinicalTrials.gov. Retrieved 2017-05-22.
