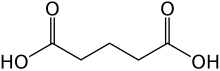
Glutaric acid
| |

| |
| Names | |
|---|---|
| IUPAC name
pentanedioic acid
| |
| Other names
Propane-1,3-dicarboxylic acid; 1,3-propanedicarboxylic acid; pentanedioic acid; n-Pyrotartaric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.471 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H8O4 | |
| Molar mass | 132.12 g/mol |
| Melting point | 95 to 98 °C |
| Boiling point | 200 °C/20 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glutaric acid is the organic compound with the formula C3H6(COOH)2 . Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50%.
Preparation
Glutaric acid can be prepared by the ring-opening of butyrolactone with potassium cyanide to give the mixed potassium carboxylate-nitrile that is hydrolyzed to the diacid.[1] Alternatively hydrolysis, followed by oxidation of dihydropyran gives glutaric acid. It can also be prepared from reacting 1,3-dibromopropane with sodium or potassium cyanide to obtain the dinitrile, followed by hydrolysis.
Uses
1,5-Pentanediol, a common plasticizer and precursor to polyesters is manufactured by hydrogenation of glutaric acid and its derivatives.[2]
Glutaric acid itself has been used in the production of polymers such as polyester polyols, polyamides. The odd number of carbon atoms (i.e. 5) is useful in decreasing polymer elasticity.[citation needed]
References
- ^ G. Paris, L. Berlinguet, R. Gaudry, J. English, Jr. and J. E. Dayan (1963). "Glutaric Acid and Glutaramide". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 496. - ^ Peter Werle and Marcus Morawietz "Alcohols, Polyhydric" in Ullmann's Encyclopedia of Industrial Chemistry: 2002, Wiley-VCH: Weinheim. DOI 10.1002/14356007.a01_305
External links
