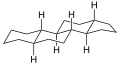
Gonane

| |

| |
| Names | |
|---|---|
| IUPAC name
2(8S,9R,10S,13S,14R)-2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17-hexadecahydro-1H-cyclopenta[a]phenanthrene
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H28 | |
| Molar mass | 232.411 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Gonane (C17), also known as perhydrocyclopenta[a]phenanthrene, is a tetracyclic hydrocarbon ring structure and the fundamental steroid nucleus.[1][2] It consists of a phenanthrene ring fused with a cyclopentane ring. Unlike steroid hormones, gonane has no methyl groups at the C10 and C13 positions and no side chain at the C17 position.[2] Because gonane has six centers of chirality, it has 64 (26) theoretically possible stereoisomers.[1] However, only a few of these stereoisomers are actually encountered with steroids.[1] The most common stereoisomers of gonane are 5α-gonane and 5β-gonane.
-
5α-Gonane
-
5β-Gonane
-
5α-Gonane, side-perspective view
-
5β-Gonane, side-perspective view
Estrane (C18) is the 13β-methyl variant of gonane, androstane (C19) is the 10β,13β-dimethyl variant of gonane, and pregnane (C21) is the 10β,13β-dimethyl, 17β-ethyl variant of gonane.[3][4]
References
- ^ a b c Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9.
- ^ a b James G. Speight (24 December 2010). Handbook of Industrial Hydrocarbon Processes. Gulf Professional Publishing. pp. 474–. ISBN 978-0-08-094271-1.
- ^ D. Sriram (1 September 2010). Medicinal Chemistry. Pearson Education India. pp. 594–. ISBN 978-81-317-3144-4.
- ^ Etienne-Emile Baulieu; Paul A. Kelly (30 November 1990). Hormones: From Molecules to Disease. Springer Science & Business Media. pp. 391–. ISBN 978-0-412-02791-8.