Herpesvirus glycoprotein B
This article may be too technical for most readers to understand. (November 2011) |
| Herpesvirus Glycoprotein B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
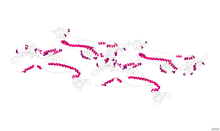 Crystallographic structure of glycoprotein B from herpes simplex virus type 1.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Glycoprotein_B | ||||||||
| Pfam | PF00606 | ||||||||
| InterPro | IPR000234 | ||||||||
| OPM superfamily | 117 | ||||||||
| OPM protein | 3nw8 | ||||||||
| |||||||||
Herpesvirus glycoprotein B is a viral glycoprotein that is involved in the viral cell entry of Herpes simplex virus (HSV). Herpesviruses have an envelope and an outer lipid bilayer which contains twelve surface glycoproteins. For infectivity to be attained, the double stranded DNA genome of HSV must enter the host cell through means of fusion of its envelope with the cellular membrane or via endocytosis. Other viral glycoproteins involved in the process of viral cell entry include gC,gB,gD,gH, and gL, but only gC,gB,gD, and gH are required for the fusion of the HSV's envelope with the cellular membrane. It can be noted that all herpesviruses have glycoproteins gB, gH, and gL.[2]
Structure
The herpesvirus glycoprotein B is a type-1 transmembrane protein with a signal sequence at its N terminus.[2] The crystal structure of herpes simplex virus (HSV) type-1 and Epstein-Barr virus glycoprotein B ectodomains were solved as a trimer, revealing five structural domains (I-V).[3][4] Domain I contains two internal fusion loops, thought to insert into the cellular membrane during virus-cell fusion.[5] In HSV, domain II is hypothesized to interact with another herpesvirus glycoprotein, gH/gL, during the fusion process.[6] Domain III consists of a structurally important elongated alpha helix, while domain IV is hypothesized to interact with cellular receptors.[7] Finally, domain V acts in conjunction with domain I during protein-lipid interactions. In HSV, neutralizing monoclonal antibodies map to structural domains I, II, IV and V.[7] Due to its unique structure, herpesvirus glycoprotein B (along with vesicular stomatitis virus glycoprotein G and baculovirus gp64) belongs to a new class of viral membrane fusion glycoproteins, class III.[3]
Function
The herpesvirus glycoprotein B is the most highly conserved of all surface glycoproteins and acts primarily as a fusion protein. The precise functions of gB and gH/gL are unknown but they are required for viral entry into the cell and constitute the core fusion machinery. The claim that gB is involved in fusion comes from the notable syncytial phenotype caused by certain mutations within the cytoplasmic domain of glycoprotein B,[8] as well as its structural homology to other viral fusion proteins.[3]
References
- ^ PDB: 3NW8; Stampfer SD, Lou H, Cohen GH, Eisenberg RJ, Heldwein EE (December 2010). "Structural basis of local, pH-dependent conformational changes in glycoprotein B from herpes simplex virus type 1". J. Virol. 84 (24): 12924–33. doi:10.1128/JVI.01750-10. PMC 3004323. PMID 20943984.
- ^ a b Pereira L, Ali M, Kousoulas K, Huo B, Banks T (September 1989). "Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues". Virology. 172 (1): 11–24. doi:10.1016/0042-6822(89)90102-5. PMID 2475970.
- ^ a b c Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC (July 2006). "Crystal structure of glycoprotein B from herpes simplex virus 1". Science. 313 (5784): 217–20. doi:10.1126/science.1126548. PMID 16840698.
- ^ Backovic M, Longnecker R, Jardetzky TS (February 2009). "Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B". Proc. Natl. Acad. Sci. U.S.A. 106 (8): 2880–5. doi:10.1073/pnas.0810530106. PMC 2650359. PMID 19196955.
- ^ Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, Cohen GH (July 2009). "Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops". J. Virol. 83 (13): 6825–36. doi:10.1128/JVI.00301-09. PMC 2698560. PMID 19369321.
- ^ Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ (April 2010). "Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion". J. Virol. 84 (8): 3825–34. doi:10.1128/JVI.02687-09. PMC 2849501. PMID 20130048.
- ^ a b Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH (April 2007). "Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions". J. Virol. 81 (8): 3827–41. doi:10.1128/JVI.02710-06. PMC 1866100. PMID 17267495.
- ^ "datasheets.scbt.com" (PDF).
Further reading
- "Santa Cruz HSV Glycoprotein Datasheet" (PDF). Santa Cruz Biotechnology, Inc.
