Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds.[1] Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis."[2]
Scope and mechanism
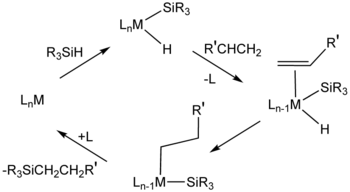
Hydrosilylation of alkenes represents a commercially important method for preparing organosilicon compounds. The process is mechanistically similar to the hydrogenation of alkenes. In fact, similar catalysts are sometimes employed for the two catalytic processes.
The prevalent mechanism, called the Chalk-Harrod mechanism, assumes an intermediate metal complex that contains a hydride, a silyl ligand (R3Si), and the alkene substrate. Oxidative addition proceeds by the intermediacy of a sigma-complex, wherein the Si-H bond is not fully broken.
Hydrosilylation of alkenes usually proceeds via anti-Markovnikov addition, i.e., silicon is placed at the terminal carbon when hydrosilylating a terminal alkene[1] Variations of the Chalk-Harrod mechanism exist. Some cases involve insertion of alkene into M-Si bond followed by reductive elimination, the opposite of the sequence in the Chalk-Harrod mechanism. In certain cases, hydrosilylation results in vinyl or allylic silanes resulting from beta-hydride elimination.[3]
Alkynes also undergo hydrosilylation, e.g., the addition of triethylsilane to diphenylacetylene:[4]
- Et3SiH + PhC≡CPh → Et3Si(Ph)C=CH(Ph)
Asymmetric hydrosilylation
Using chiral phosphines as spectator ligands, catalysts have been developed for catalytic asymmetric hydrosilation. A well studied reaction is the addition of trichlorosilane to styrene to give 1-phenyl-1-(trichlorosilyl)ethane:
- Cl3SiH + PhCH=CH2 → (Ph)(CH3)CHSiCl3
Nearly perfect enantioselectivities (ee's) can be achieved using palladium catalysts supported by binaphthyl-substituted monophosphine ligands.[5]
Surface hydrosilylation
Silicon wafers can be etched in hydrofluoric acid (HF) to remove the native oxide and form a hydrogen-terminated silicon surface. The hydrogen-terminated surfaces undergo hydrosilation with unsaturated compounds (such as terminal alkenes and alkynes), to form a stable monolayer on the surface. For example:
- Si-H + H2C=CH(CH2)7CH3 → Si-CH2CHH-(CH2)7CH3
The hydrosilylation reaction can be initiated with UV light at room temperature or with heat (typical reaction temperature 120-200 °C), under moisture- and oxygen-free conditions.[6] The resulting monolayer, which is stable and inert, inhibits oxidation of the base silicon layer, relevant to various device applications.[7]
Catalysts

Prior to the introduction of platinum catalysts by Speier, hydrosilylation was not practiced widely. A peroxide-catalyzed process was reported in academic literature in 1947,[8] but the introduction of Speier's catalyst (H2PtCl6) was a major breakthrough.
Karstedt’s catalyst was later introduced. It is a lipophilic complex that is soluble in the organic substrates of industrial interest.[9] Complexes and compounds that catalyze hydrogenation are often effective catalysts for hydrosilylation, e.g. Wilkinson's catalyst.
References
- ^ a b "Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009. doi:10.1007/978-1-4020-8172-9
- ^ Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; Lang, J.; Kreuzer, T.; Knödler, A.; Starz, K. A.; Dermann, K.; Rothaut, J.; Drieselman, R. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
- ^ Troegel, D.; Stohrer, J. (2011). "Recent Advances and Actual Challenges in Late Transition Metal Catalyzed Hydrosilylation of Olefins from an Industrial Point of View". Coord. Chem. Rev. 255: 1440-1459. doi:10.1016/j.ccr.2010.12.025.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ James L. Fry, Ronald J. Rahaim Jr., Robert E. Maleczka, Jr. "Triethylsilane", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2007. doi:10.1002/047084289X.rt226.pub2
- ^ Hayashi, T.; Yamasaki, K. (2007). "C–E Bond Formation through Asymmetric Hydrosilylation of Alkenes". In Crabtree, Robert H.; Mingos, D. Michael P. (eds.). Comprehensive Organometallic Chemistry III. Amsterdam: Elsevier. doi:10.1016/B0-08-045047-4/00140-0. ISBN 978-0-08-045047-6.
- ^ "Photoreactivity of Unsaturated Compounds with Hydrogen-Terminated Silicon (111)," R. L. Cicero, M. R. Linford, C. E. D. Chidsey, Langmuir 16, 5688-5695 (2000).
- ^ "Direct electrical detection of DNA Hybridization at DNA-modified silicon surfaces," W.Cai, J. Peck, D. van der Weide, and R.J. Hamers, Biosensors and Bioelectronics 19, 1013-1019 (2004).
- ^ Sommer, L.; Pietrusza, E.; Whitmore, F. "Peroxide-catalyzed addition of trichlorosilane to 1-octene". J. Am. Chem. Soc. 69 (1): 188. doi:10.1021/ja01193a508.
- ^ C. Elschenbroich, Organometallics (2006) Wiley and Sons-VCH: Weinheim. ISBN 978-3-527-29390-2
Further reading
- Applied homogeneous catalysis with organometallic compounds : a comprehensive handbook : applications, developments. Boy Cornils; W A Herrmann. Publisher: Weinheim ; New York : Wiley-VCH, ©2000.
- Comprehensive handbook on hydrosilylation. Bogdan Marciniec. Publisher: Oxford [u.a.] : Pergamon Press, 1992.
- Rhodium complexes as hydrosilylation catalysts. N.K. Skvortsov. // Rhodium Express. 1994. No 4 (May). P. 3 - 36 (Eng). [1] ISSN 0869-7876
Additional specialized literature
- "Alkyl Monolayers on Silicon Prepared from 1-Alkenes and Hydrogen-Terminated Silicon," M. R. Linford, P. Fenter, P. M. Eisenberger and C. E. D. Chidsey, J. Am. Chem. Soc. 117, 3145-3155 (1995).
- "Synthesis and characterization of DNA-modified Si(111) Surfaces," T. Strother, W. CAi, X. Zhao, R.J. Hamers, and L.M. Smith, J. Am. Chem. Soc. 122, 1205-1209 (2000).
- "T. Strother, R.J. Hamers, and L.M. Smith, "Surface Chemistry of DNA Covalent Attachment to the Silicon(100) Surface". Langmuir, 2002, 18, 788-796.
- "Covalently Modified Silicon and Diamond Surfaces: Resistance to Non-Specific Protein Adsorption and Optimization for Biosensing," T.L. Lasseter, B.H. Clare, N.L. Abbott, and R.J. Hamers. J. Am. Chem. Soc. 2004, 126, 10220-10221.
