Iron-56
| General | |
|---|---|
| Symbol | 56Fe |
| Names | iron-56, 56Fe, Fe-56, Iron-56 |
| Protons (Z) | 26 |
| Neutrons (N) | 30 |
| Nuclide data | |
| Natural abundance | 91.754% |
| Isotope mass | 55.9349375 Da |
| Spin | 0+ |
| Excess energy | −60601.003 keV |
| Binding energy | 492253.892 keV |
| Isotopes of iron Complete table of nuclides | |
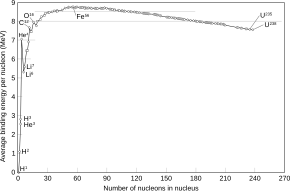
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56.
Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei.[1]
Nickel-62, a relatively rare isotope of nickel, has a higher nuclear binding energy per nucleon; this is consistent with having a higher mass per nucleon because nickel-62 has a greater proportion of neutrons, which are slightly more massive than protons. See the nickel-62 article for more information regarding the ordering of binding energy per nucleon, and mass-per-nucleon, for various nuclides.
Thus, light elements undergoing nuclear fusion and heavy elements undergoing nuclear fission release energy as their nucleons bind more tightly, and the resulting nuclei approach the maximum total energy per nucleon, which occurs at 62Ni. However, during nucleosynthesis in stars the competition between photodisintegration and alpha capturing causes more 56Ni to be produced than 62Ni (56Fe is produced later in the star's ejection shell as 56Ni decays). This means that as the Universe ages, more matter is converted into extremely tightly bound nuclei, such as 56Fe. This progression of matter towards iron and nickel is one of the phenomena responsible for the heat death of the universe.
Production of these elements has decreased considerably from what it was at the beginning of the stelliferous era.[citation needed]
See also
References
- J. R. de Laeter; J. K. Böhlke; P. De Bièvre; H. Hidaka; H. S. Peiser; K. J. R. Rosman; P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
