Kaminsky catalyst
A Kaminsky catalyst is a catalytic system for alkene polymerization.[1] Kaminsky catalysts are based on metallocenes of group 4 metals (Ti, Zr, Hf) activated with methylaluminoxane (MAO). These and other innovations have inspired development of new classes of catalysts that in turn led to commercialization of novel engineering polyolefins.[2]
Catalyst development
Prior to Kaminsky, titanium chlorides supported on various materials were widely used (and still are) as heterogeneous catalysts for alkene polymerization. These halides are typically activated by treatment with trimethylaluminium. Kaminsky discovered that titanocene and related complexes emulated some aspects of these Ziegler-Natta catalysts but with low activity. He subsequently found that high activity could be achieved upon activation of these metallocenes with methylaluminoxane (MAO). The MAO serves two roles: (i) alkylation of the metallocene halide and (ii) abstraction of an anionic ligand (chloride or methyl) to give an electrophilic catalyst with a labile coordination site.[1][3]
Ligand design
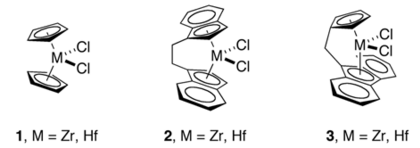
Kaminsky's discovery of well-defined, high activity homogeneous catalysts led to many innovations in the design of novel cyclopentadienyl ligands. These innovations include ansa-metallocenes, Cs-symmetric fluorenyl-Cp ligands,[4] constrained geometry catalysts,[5] Some Kaminsky-inspired catalysts use of chiral metallocenes that have bridged cyclopentadienyl rings. These innovations made possible highly stereoselective (or stereoregular) polymerization of α-olefins, some of which have been commercialized.[2]
References
- ^ a b Walter Kaminsky (1998). "Highly Active Metallocene Catalysts For Olefin Polymerization". Journal of the Chemical Society, Dalton Transactions: 1413–1418. doi:10.1039/A800056E.
- ^ a b Klosin, J.; Fontaine, P. P.; Figueroa, R., (2015). "Development of Group Iv Molecular Catalysts for High Temperature Ethylene-Α-Olefin Copolymerization Reactions". Accounts of Chemical Research. 48: 2004–2016. doi:10.1021/acs.accounts.5b00065.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Chen, E. Y.-X.; Marks, T. J. (2000). "Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships". Chem. Rev. 100 (4): 1391–1434. doi:10.1021/cr980462j. PMID 11749269.
- ^ Ewen, J. A.; Jones, R. L.; Razavi, A.; Ferrara, J. D. (1988). "Syndiospecific propylene polymerizations with Group IVB metallocenes". Journal of the American Chemical Society. 110: 6255–6256. doi:10.1021/ja00226a056.
- ^ Shapiro, P. J.; Bunel, E.; Schaefer, W. P.; Bercaw, J. E. "Scandium Complex [{(η5-C5Me4)Me2Si(η1-NCMe3)}(PMe3)ScH]2: A Unique Example of a Single-Component α-Olefin Polymerization Catalyst" Organometallics 1990, volume 9, pp. 867−869.

