Leucines
Appearance
The leucines are primarily the four isomeric amino acids: leucine, isoleucine, tert-leucine and norleucine. Being compared with the four butanols, they could be classified as butyl-substituted glycines; they represent all four possible variations.
Leucine and isoleucine belong to the proteinogenic amino acids; the others are non-natural.
Isomers
Including the stereoisomers, six further isomers could be added: D-leucine, D-isoleucine, L-allo-isoleucine, D-allo-isoleucine, D-tert-leucine and D-norleucine.
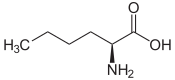
| Leucines | ||||
| Name | L-Leucine | L-Isoleucine | L-tert-Leucine (Terleucine) | L-Norleucine |
| Other names | 2-Amino-4-methylpentanoic acid, Isobutylglycine |
2-Amino-3-methylpentanoic acid, sec-Butylglycine |
2-Amino-3,3-dimethylbutanoic acid, tert-Butylglycine |
2-Amino-hexanoic acid, n-Butylglycine |
| Structure | 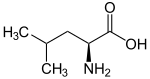 |
 |
 |
|
| CAS-number | 61-90-5 | 73-32-5 | 20859-02-3 | 327-57-1 |
| PubChem | CID 6106 from PubChem | CID 791 from PubChem | CID 164608 from PubChem | CID 21236 from PubChem |
| Molecular formula | C6H13NO2 | |||
| Molar mass | 131.18 g/mol | |||
Derivatives
Cycloleucine could be classified as a cyclic derivative of norleucine. With a cyclopentane-ring, it has two hydrogen atoms fewer and thus is not an isomer. The α-carbon atom is not a stereocenter.

