Leyden jar: Difference between revisions
SoxBot III (talk | contribs) m Reverting possible test edit(s) by 24.174.150.199 to older version. Was this a mistake? (BOT EDIT) |
|||
| Line 3: | Line 3: | ||
The '''Leyden jar''' is a device that "stores" [[static electricity]] between two electrodes on the inside and outside of a jar. It was invented in 1745 by [[Pieter van Musschenbroek]] (1692–1761), in [[Leiden]], The Netherlands. It was the original form of the [[capacitor]]. The Leyden jar was used to conduct many early experiments in [[electricity]], and its discovery was of fundamental importance in the study of electricity. Previously, researchers had to resort to insulated conductors of large dimensions to store charge. The Leyden jar provided a much more compact alternative. |
The '''Leyden jar''' is a device that "stores" [[static electricity]] between two electrodes on the inside and outside of a jar. It was invented in 1745 by [[Pieter van Musschenbroek]] (1692–1761), in [[Leiden]], The Netherlands. It was the original form of the [[capacitor]]. The Leyden jar was used to conduct many early experiments in [[electricity]], and its discovery was of fundamental importance in the study of electricity. Previously, researchers had to resort to insulated conductors of large dimensions to store charge. The Leyden jar provided a much more compact alternative. |
||
== |
|||
== Description == |
|||
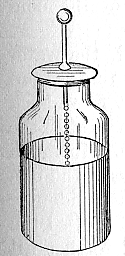
A typical design consists of a [[glass]] jar with conducting metal foil coating the inner and outer surfaces. The foil coatings stop short of the mouth of the jar, to prevent the charge from arcing between the foils. A rod [[electrode]] projects through the mouth of the jar, electrically connected by some means (usually a chain) to the inner foil, to allow it to be charged. The jar is charged by an [[electrostatic generator]], or other source of electric charge, connected to the inner electrode while the outer foil is [[grounded]]. The inner and outer surfaces of the jar store equal but opposite charges. |
|||
[[Image:Leyden_jar_cutaway.png|thumb|left|Cross-section diagram from about 1900 showing construction.]] |
|||
The original form of the device was just a glass bottle partially filled with water, with a metal wire passing through a cork closing it. The role of the outer plate was provided by the hand of the experimenter. Soon it was found that it was better to coat the exterior of the jar with metal foil (Watson, 1746), leaving the (accidentally) impure water inside acting as a conductor, connected by a chain or [[wire]] to an external terminal, a sphere to avoid losses by [[corona discharge]]. It was initially believed that the charge was stored in the water. [[Benjamin Franklin]] investigated the Leyden jar, and concluded that the charge was stored in the glass, not in the water, as others had assumed. We now know that the charge is actually stored in the conductors, but only in a thin layer along the facing surfaces that touch the glass, or [[dielectric]]. The charge may leak to the surface of the dielectric if contact is imperfect and the electric field is intense enough. Because of this, the fluid inside can be replaced with a metal foil lining. Early experimenters found that the thinner the [[dielectric]], the closer the plates, and the greater the surface, the greater the amount of charge that could be stored at a given [[voltage]]. |
|||
Further developments in electrostatics revealed that the dielectric material was not essential, but increased the storage capability ([[capacitance]]) and prevented arcing between the plates. Two plates separated by a small distance also act as a capacitor, even in [[vacuum]]. |
|||
[[Image:Leyden_unit_jar.png|thumb|Measuring Leyden jar.]] |
|||
[[Image:Leidse_flessen_Museum_Boerhave_december_2003 2.jpg|thumb|A "battery" of four Leyden jars, [[Museum Boerhaave]], Leiden [http://www.museumboerhaave.nl/]. Named by [[Benjamin Franklin]] , derived from a "[[Artillery battery|battery of cannon]]".]] |
|||
''Originally'', the amount of [[capacitance]] was measured in number of 'jars' of a given size, or through the total coated area, assuming reasonably standard thickness and composition of the glass. A typical Leyden jar of one [[pint]] size has a capacitance of about 1 [[farad|nF]]. |
|||
== History == |
== History == |
||
Revision as of 13:43, 7 January 2009
The Leyden jar is a device that "stores" static electricity between two electrodes on the inside and outside of a jar. It was invented in 1745 by Pieter van Musschenbroek (1692–1761), in Leiden, The Netherlands. It was the original form of the capacitor. The Leyden jar was used to conduct many early experiments in electricity, and its discovery was of fundamental importance in the study of electricity. Previously, researchers had to resort to insulated conductors of large dimensions to store charge. The Leyden jar provided a much more compact alternative.
==
History
The ancient Greeks already knew that pieces of amber could be rubbed, becoming electrified and attracting light particles. This is the triboelectric effect, mechanical separation of charge in a dielectric. It is why the word "electricity" was made from the Greek word ηλεκτρον ("elektron", amber).
Around 1650, Otto von Guericke built a crude friction generator — a sulphur ball that rotated on a shaft. When Guericke held his hand against the ball and turned the shaft quickly, a static electric charge built up. This experiment inspired the development of several forms of "friction machines", that greatly helped in the study of electricity.
The Leyden jar was first invented by German scientist and jurist Ewald Georg von Kleist, who in 1745 found a method of storing large amounts of electric charge. He lined a glass jar with silver foil, and charged the foil with a friction machine. Kleist was convinced that a substantial charge could be collected when he received a significant shock from the device. This Kleistian jar became known as the Leyden jar because in 1746, Dutch scientist Pieter van Musschenbroek of the University of Leiden, independently made the same discovery. Musschenbroek made the storage jar known to the scientific world, hence the jar was named after Leiden, the home town of the university. Daniel Gralath was the first to combine several jars in parallel into a "battery" to increase the total possible stored charge. [1][2] By the middle of the 19th century, the Leyden jar had become common enough for writers to assume their readers knew of and understood its basic operation.[3] By the early 20th century, improved dielectrics and the need to reduce their size and inductance for use in the new technology of radio caused the Leyden jar to evolve into the modern compact form of capacitor.
The "dissectible Leyden jar" myth
A popular, but misleading, demonstration with a Leyden jar involves taking one apart after it has been charged and showing that the energy is stored on the dielectric, not the plates. The first documented instance of this demonstration is in a 1749 letter by Benjamin Franklin. [4]
The jar in the demonstration is constructed out of a dielectric cup nested between two snugly fitting metal cups. When the jar is charged with a high voltage and carefully dismantled, it is discovered that all the parts may be freely handled without discharging the jar. If the pieces are re-assembled, a large spark may still be obtained.
This demonstration shows that the charge has been transferred to the surface of the dielectric, and is not on the metal conductors. When the jar is taken apart, simply touching the cup does not give enough contact area to remove all the charge. The conductors normally provide this surface area.
When not properly explained, this promoted the myth that capacitors store their charge on their dielectric. However, this behaviour is not typical of capacitors, and does not happen at low voltages. In a typical capacitor, the charge is on the surface of the conductors. The transfer of charge to the dielectric in the above experiment results from the high voltages present when the conductors are separated from the dielectric, which redeposits charge onto the surface of the dielectric by means of a corona discharge at the edges of the plates as they slide along the dielectric during the disassembly. If the experiment were performed in a highly insulating fluid (such as mineral oil) instead of air, the effect would no longer be present, or would require higher voltages.[5]
See also
References
- ^ The Leyden Jar Discovered — World Wide School
- ^ The term "battery" was coined by Benjamin Franklin, who likened it to a battery of cannon (cannons grouped in a common place). The term was later used for arrangements of multiple electrochemical cells, the modern meaning of battery.
- ^ The Leyden Ball
- ^ Letter IV: Benjamin Franklin to Peter Collinson, April 29, 1749 (Bigelow vol II p. 237-253) (PDF containing extracts)
- ^ Capacitor complaints — William J. Beaty, 1996
External links
- Leyden Jar - Interactive Java Tutorial National High Magnetic Field Laboratory
