Methyl cellulose
| |
| Names | |
|---|---|
| Other names
Cellulose, methyl ether; methylated cellulose; methylcellulose; E461
| |
| Identifiers | |
| ECHA InfoCard | 100.115.188 |
| E number | E461 (thickeners, ...) |
CompTox Dashboard (EPA)
|
|
| Properties | |
| variable | |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl cellulose (or methylcellulose) is a chemical compound derived from cellulose. It is a hydrophilic white powder in pure form and dissolves in cold (but not in hot) water, forming a clear viscous solution or gel. It is sold under a variety of trade names and is used as a thickener and emulsifier in various food and cosmetic products, and also as a treatment of constipation. Like cellulose, it is not digestible, not toxic, and not allergenic.
Chemistry
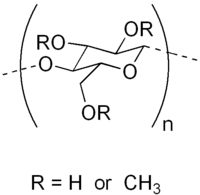
Chemically, methyl cellulose is a methyl ether of cellulose, arising from substituting the hydrogen atoms of some of cellulose's hydroxyl groups -OH with methyl groups -CH3, forming -OCH3 groups.
Different kinds of methyl cellulose can be prepared depending on the number of hydroxyl groups so substituted. Cellulose is a polymer consisting of numerous linked glucose molecules, each of which exposes three hydroxyl groups. The Degree of Substitution (DS) of a given form of methyl cellulose is defined as the average number of substituted hydroxyl groups per glucose. The theoretical maximum is thus a DS of 3.0, however more typical values are 1.3–2.6.
Different methyl cellulose preparations can also differ in the average length of their polymer backbones.
Methyl cellulose does not occur naturally and is synthetically produced by heating cellulose with caustic solution (e.g. a solution of sodium hydroxide) and treating it with methyl chloride.
Solubility and temperature
Methyl cellulose dissolves in cold water. Higher DS-values result in lower solubility, because the polar hydroxyl groups are masked. The chemical is not soluble in hot water, which has the paradoxical effect that heating a saturated solution of methyl cellulose will turn it solid, because methyl cellulose will precipitate out. The temperature at which this occurs depends on DS-value, with higher DS-values giving lower precipitation temperatures.
Preparing a solution of methyl cellulose with cold water is difficult however: as the powder comes into contact with water, a gluey layer forms around it, and the inside remains dry. A better way is to first mix the powder with hot water, so that the methyl cellulose particles are well dispersed in the water, and cool down this dispersion while stirring, leading to the dissolution of those particles.
Uses
Methyl cellulose has an extremely wide range of uses, of which several are described below.
Stem cell differentiation
Methylcellulose is used in the most common approaches to quantify multi-lineage- or single lineage-committed hematopoietic progenitors, called colony-forming cells (CFCs) or colony-forming units (CFUs), in combination with culture supplements that promote their proliferation and differentiation and allow the clonal progeny of a single progenitor cell to stay together and thus form a colony of more mature cells. MethoCult is one such methylcellulose-based media.
Scientifically Advanced Cookery
Methyl cellulose, as a gel, has the unique property of setting when hot and melting when cold. This technique is currently being developed at the University of Nottingham.
Thickener and emulsifier
Methyl cellulose is often added to hair shampoos, tooth pastes and liquid soaps, to generate their characteristic thick consistency. This is also done for foods, for example ice cream or croquette. Methyl cellulose is also an important emulsifier, preventing the separation of two mixed liquids.
The E number of methyl cellulose as food additive is E461.
Treatment of constipation
When eaten, methyl cellulose is not absorbed by the intestines but passes through the digestive tract undisturbed. It attracts large amounts of water into the colon, producing a softer and bulkier stool. It is used to treat constipation, diverticulosis, hemorrhoids and irritable bowel syndrome. It should be taken with sufficient amounts of fluid to prevent dehydration.
Because it absorbs water and potentially toxic materials and increases viscosity, it can also be used to treat diarrhea.
A well-known trade name of methyl cellulose when used as a drug is Citrucel by GlaxoSmithKline, but generic versions are also widely available.
Lubricant
Methyl cellulose is used as a variable viscosity personal lubricant; it is the main ingredient in K-Y Jelly.
Artificial tears and saliva
Solutions containing methyl cellulose or similar cellulose derivatives (see below) are used as substitute for tears or saliva if the natural production of these fluids is disturbed.
Bacterial Motility Inhibitor
Aqueous methyl cellulose solutions have been used to slow bacterial cell motility for closer inspection. Changing the amount of methyl cellulose in solution allows one to adjust the solution's viscosity.
Paper and textile sizing
Methyl cellulose is used as sizing in the production of papers and textiles. It protects the fibers from absorbing water or oil.
Glue and binder
Methyl cellulose can be employed as a mild glue which can be washed away with water. This is used for example in the fixation of delicate pieces of art.
Methyl cellulose is the main ingredient in many wallpaper pastes.
It is also used as a binder in pastel crayons and also as a binder in medications.
Methyl cellulose is used in book conservation to loosen and clean off old glue from spines and bookboards.
Construction materials
Methyl cellulose finds a major application as a performance additive in construction materials. It is added to mortar dry mixes to improve the mortar's properties such as workability, open and adjustment time, water retention, viscosity, adhesion to surfaces etc. Construction grade methyl cellulose is to not to be identified with food and pharmaceutical grade methyl cellulose and hydroxypropyl methyl cellulose, since it may be cross-linked with glyoxal for easy dispersion in water.
The construction materials can be cement based or gypsum based. Notable examples of dry mixture mortars which utilize methyl cellulose include: tile adhesives, EIFS, insulating plasters, hand-trowed and machine sprayed plaster, stucco, self-leveling flooring, extruded cement panels, skim coats, joint & crack fillers, and tile grouts. Typical usage is about 0.2% ~ 0.5% of total dry powder weight for dry mixture
Derivatives of methyl cellulose, which improve upon the performance characteristics, include hydroxypropyl methyl cellulose (HPMC) and hydroxyethyl methyl cellulose (HEMC). These derivatives typically improve the characteristics such as water retention, vertical surface slip-resistance, open time, etc.
Manufacturers of such construction grade methyl cellulose include SE Tylose, Dowwolff, Shin-Etsu, Samsung Fine Chemicals, Hercules Aqualon, and various smaller manufacturers.
Cell culture/virology
Methyl cellulose is also used in cell culture to study viral replication. Methyl cellulose is dissolved in the same nutrient containing media that cells are normally grown in. A single layer of cells are grown on a flat surface, then infected with a virus for a short time. The strength of the viral sample used will determine how many cells get infected during this time. The thick methyl cellulose media is then added on top of the cells in place of normal liquid media. As the viruses replicate in the infected cells they are able to spread between cells whose membrances touch each other, but are trapped when they enter the methyl cellulose. Only cells closely neighboring an infected cell will become infected and die. This leaves small regions of dead cells called plaques in a larger background of living uninfected cells. The number of plaques formed is determined by the strength of the original sample.
Nutritional Supplement Capsules
Methyl cellulose is also used in the manufacture of vegetarian capsules in nutritional supplements, its edible and non-toxic properties provide a safe alternative to the use of gelatin.
Special effects
The slimy, gooey appearance of an appropriate preparation of methyl cellulose with water, in addition to its non-toxic, non-allergenic, and edible properties, makes it popular for use in special effects for motion pictures and television wherever vile slimes must be simulated. In the film Ghostbusters, for example, the gooey substance that supernatural entities used to “slime” the Ghostbusters was mostly a thick water solution of methyl cellulose.
Methyl cellulose is often used in the pornographic industry to simulate semen in large quantity, in order to shoot movies related to bukkake fetish. It is preferable to food-based fake semen (e.g., condensed milk) because this last solution can often cause problems, especially when the ingredient used contains sugar. Sugar is thought to encourage yeast infection when it is injected into the vagina.
See also
References
- Cathleen Baker (1984). Methylcellulose & Sodium Carboxymethylcellulose: An Evaluation for Use in Paper Conservation through Accelerated Aging. Preprints of the Contributions to the Paris Congress, 2-8 September 1984: Adhesives and Consolidants, pp. 55-59. International Institute of Conservation.
- Robert L. Feller and Myron H. Wilt (1990). Evaluation of Cellulose Ethers for Conservation (free fulltext). Getty Conservation Institute.
- Pijper A (1947). "Methylcellulose and Bacterial Motility". J. Bacteriol. 53 (3): 257–69. PMC 518306. PMID 16561270.
{{cite journal}}: Unknown parameter|month=ignored (help)
