Nitrogen-13
| General | |
|---|---|
| Symbol | 13N |
| Names | nitrogen-13, 13N, N-13 |
| Protons (Z) | 7 |
| Neutrons (N) | 6 |
| Nuclide data | |
| Half-life (t1/2) | 9.97 min |
| Parent isotopes | 13O (Electron capture) |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| Beta + | 1.2003 |
| Isotopes of nitrogen Complete table of nuclides | |
Nitrogen-13 is a radioisotope of nitrogen used in positron emission tomography (PET). It has a half life of a little under ten minutes, so it must be made at the PET site. A cyclotron may be used for this purpose.
Nitrogen-13 is used to tag ammonia molecules for PET myocardial perfusion imaging.
Production
- 1H + 16O → 13N + 4He
The proton must be accelerated to a kinetic energy of about 5.55 MeV or a little more.
The reaction is endothermic (i.e. the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass).This is one reason why the proton needs to carry extra energy to produce the nuclear reaction.
The energy difference is actually 5.22 MeV, but if the proton only supplied this energy the reactants would be formed with no kinetic energy. As momentum must be conserved, the true energy that needs to be supplied by the proton is given by:
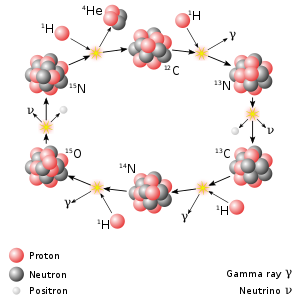
Nitrogen-13 plays a significant role in the CNO cycle, which is the dominant source of energy in stars heavier than the sun.[1]
External links
- PET site of the University of Melbourne
References
- ^ Phillips, A.C. (1994). The Physics of Stars. John Wiley & Sons. ISBN 0-471-94057-7.

