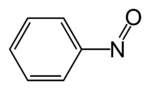
Nitrosobenzene
| |

| |
| Names | |
|---|---|
| IUPAC name
Nitrosobenzene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.721 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H5NO | |
| Molar mass | 107.112 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 65 to 69 °C (149 to 156 °F; 338 to 342 K) |
| Boiling point | 59 °C (138 °F; 332 K) (at 18 mmHg) |
| Low | |
| Solubility in other solvents | Sol. in organic solvents |
| -59.1·10−6 cm3/mol | |
| Structure | |
| N is sp2 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| Related compounds | |
Related compounds
|
Nitrobenzene Aniline |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. It is a bright blue species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.
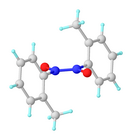
Monomer-dimer equilibrium
Nitrosobenzene and other nitrosoarenes typically participate in a monomer-dimer equilibrium. The dimers are often favored in the solid state, whereas the deeply colored monomers are favored in dilution solution or at higher temperatures. The dimers can be formulated as Ph(O-)N+=N+(O-)Ph. They exist as cis- and trans-isomers. The dimers are sometimes called azobenzenedioxides. The cis-trans isomerization occurs via the intermediacy of the monomer.[1]
Preparation
Nitrosobenzene was first prepared by Adolf von Baeyer by the reaction of diphenylmercury and nitrosyl bromide:[3]
- (C6H5)2Hg + BrNO → C6H5NO + C6H5HgBr
A modern synthesis entails reduction of nitrobenzene to phenylhydroxylamine (C6H5NHOH) which is then oxidized by sodium dichromate (Na2Cr2O7).[4]
Nitrosobenzene can also be prepared by oxidation of aniline using peroxymonosulfuric acid (Caro's acid)[5] or Oxone.[6] It is usually purified by steam distillation, where it comes over as a green liquid that solidifies to a colorless solid.
Characteristic reactions
Nitrosobenzene undergoes Diels-Alder reactions with dienes.[7] Condensation with anilines affords azobenzene derivatives in a reaction known as the Mills reaction.[8] Reduction of nitrosobenzene produces aniline.
Most characteristically, nitrosobenzene condenses with active methylene groups, such as those of malonic esters and benzyl cyanide. Benzylcyanide (PhCH2CN) gives the imine (PhC(CN)=NPh) in a reaction known as the Ehrlich-Sachs reaction:[9]
- Ph–CH2-CN + Ph–NO → Ph–CH(CN)–N(OH)–Ph (oxyamination adduct) → PhC(CN)=N–Ph
Sometimes condensation with active methylene compounds gives products of O-nitroso-aldol reaction:[10]
- R–CH2-CHO + Ph–NO → R–CH(CHO)–O–NHPh (aminoxylation adduct)
References
- ^ "Dimerization of Aromatic C-Nitroso Compounds". Chemical Reviews. 116: 258–286. 2016. doi:10.1021/cr500520s.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ E.Bosch (2014). "Structural Analysis of Methyl-Substituted Nitrosobenzenes and Nitrosoanisoles". J. Chem. Cryst. 98: 44. doi:10.1007/s10870-013-0489-8.
- ^ Baeyer, A. (1874). "Nitrosobenzol und Nitrosonaphtalin". Chemische Berichte. 7: 1638–1640. doi:10.1002/cber.187400702214.
- ^ G. H. Coleman, C. M. McCloskey, F. A. Stuart (1945). "Nitrosobenzene". Org. Synth. 25: 80. doi:10.15227/orgsyn.025.0080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ H. Caro (1898). Z. Angew. Chem. 11: 845ff.
{{cite journal}}: Missing or empty|title=(help) - ^ Priewisch, Beate; Rück-Braun, Karola (March 2005). "Efficient Preparation of Nitrosoarenes for the Synthesis of Azobenzenes†". The Journal of Organic Chemistry. 70 (6): 2350–2352. doi:10.1021/jo048544x. ISSN 0022-3263.
- ^ H. Yamamoto, N. Momiyama "Rich Chemistry of Nitroso Compounds" Chemical Communications 2005, pp.3514–25.
- ^ H. D. Anspon (1955). "p-Phenylazobenzoic Acid". Organic Syntheses; Collected Volumes, vol. 3, p. 711.
- ^ H. Feuer. S. Patai (ed.). New York: Wiley. pp. 278–283.
{{cite book}}:|work=ignored (help); Missing or empty|title=(help) - ^ "Asymmetric O− and N− Nitroso Aldol Reaction – an efficient access to a-oxy and a-amino carbonyl compound" (PDF).
