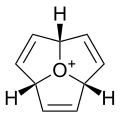
Oxatriquinane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(2as,4as,6as)-Octahydro-1H-2a1-oxacyclopenta[cd]pentalen-2a1-ium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| C9H15O | |||
| Molar mass | 139.218 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxatriquinane is an alkyl oxonium ion with formula C
9H
15O+
, remarkable for being stable in aqueous solution. It has a cyclononane backbone, with the trivalent oxygen connected to carbons 1, 4, and 7, forming three fused pentagonal rings.
Oxatriquinane was first described in 2008, and was obtained after a five-step synthesis starting from cyclononatriene.[1][2][3] At the time it had the longest C–O bond lengths (1.54 Å, C–O bonds in ethers are generally ~1.43 Å) and most acute C−O−C angles ever observed in a compound.[1]
Oxonium ions normally are strong alkylating agents and are only observed in solution as reactive intermediates or under extreme conditions. Oxatriquinane does not react with boiling water or with alcohols, thiols, halide ions, or amines, although it does react with stronger nucleophiles such as hydroxide, cyanide, and azide.[1] The ability of the oxygen to enter into a fourth covalent bonding relationship has been of some theoretical interest and was achieved using carborane acid.[4]
Analogues
Related species include oxatriquinacene,[1] the tri-unsaturated analogue, which is of interest as a possible precursor to oxaacepentalene, a neutral aromatic species. 1,4,7-tri-tert-butyloxatriquinane has also been synthesised; this compound contains significant amounts of intramolecular steric strain, resulting in further bond elongation to give C–O bond lengths of 1.622 Å, the longest recorded in any species.[5]
-
Oxatriquinacene
-
Oxaacepentalene
References
- ^ a b c d
Mark Mascal, Nema Hafezi, Nabin K. Meher, and James C. Fettinger (2008). "Oxatriquinane and Oxatriquinacene: Extraordinary Oxonium Ions". Journal of the American Chemical Society. 130 (41): 13532–13533. doi:10.1021/ja805686u. PMID 18798616.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rachel Petkewich (September 29, 2008). "Taming Alkyl Oxonium Ions: Fused tricyclic structure stabilizes famously reactive alkylating agents". Chemical and Engineering News. 86 (39): 10. doi:10.1021/cen-v086n039.p010.
- ^ Tim Reid (3 October 2008). "Oxonium ions: Ring of stability". Nature Chemistry. doi:10.1038/nchem.70.
- ^ Stoyanov, Evgenii S.; Gunbas, Gorkem; Hafezi, Nema; Mascal, Mark; Stoyanova, Irini V.; Tham, Fook S.; Reed, Christopher A. (11 January 2012). "The R3O+···H+ Hydrogen Bond: Toward a Tetracoordinate Oxadionium(2+) Ion". Journal of the American Chemical Society. 134 (1): 707–714. doi:10.1021/ja209942s.
- ^ Gunbas, Gorkem; Hafezi, Nema; Sheppard, William L.; Olmstead, Marilyn M.; Stoyanova, Irini V.; Tham, Fook S.; Meyer, Matthew P.; Mascal, Mark (18 November 2012). "Extreme oxatriquinanes and a record C–O bond length". Nature Chemistry. 4 (12): 1018–1023. doi:10.1038/nchem.1502.