PBT2
Appearance
| |
| Names | |
|---|---|
| IUPAC name
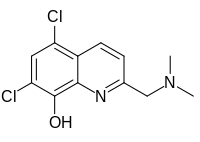
5,7-Dichloro-2-[(dimethylamino)methyl]quinolin-8-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H12Cl2N2O | |
| Molar mass | 271.14 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
PBT2 is an experimental drug candidate. It is a second-generation 8-hydroxyquinoline analog[1] intended to be a successor to clioquinol and a potential treatment of Alzheimer's disease.[2]
Clinical trials
PBT2 was the subject of phase II clinical trials for Alzheimer's disease and Huntington's disease. The results for Alzheimer's disease failed to meet its goals,[3][4] and there is no evidence that PBT2 is of any benefit in Alzheimer's dementia.[5] The drug also missed efficacy goals for Huntington's disease.[6][7]
References
- ^ Adlard, Paul A.; Cherny, Robert A.; Finkelstein, David I.; Gautier, Elisabeth; Robb, Elysia; Cortes, Mikhalina; Volitakis, Irene; Liu, Xiang; et al. (2008). "Rapid Restoration of Cognition in Alzheimer's Transgenic Mice with 8-Hydroxy Quinoline Analogs is Associated with Decreased Interstitial Aβ". Neuron. 59 (1): 43–55. doi:10.1016/j.neuron.2008.06.018. PMID 18614028.
- ^ Duce, James A.; Tsatsanis, Andrew; Cater, Michael A.; James, Simon A.; Robb, Elysia; Wikhe, Krutika; Leong, Su Ling; Perez, Keyla; et al. (2010). "Iron-Export Ferroxidase Activity of β-Amyloid Precursor Protein is Inhibited by Zinc in Alzheimer's Disease". Cell. 142 (6): 857–67. doi:10.1016/j.cell.2010.08.014. PMC 2943017. PMID 20817278.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ "Prana Biotech Plunges 76%; Drug Fails in Alzheimer Study". Bloomberg News. Mar 31, 2014.
- ^ "PBT2 Takes a Dive in Phase 2 Alzheimer's Trial". April 2014.
- ^ "There is no evidence that MPACs (PBT1 or PBT2) are of benefit in Alzheimer's dementia". Cochrane Database of Systematic Reviews: Plain Language Summaries. PubMed Health.
- ^ "Prana Bio Huntington's Disease Drug Fails Key Efficacy Hurdles". thestreet.com.
- ^ Huntington Study Group Reach2HD Investigators (2015). "Safety, tolerability, and efficacy of PBT2 in Huntington's disease: A phase 2, randomised, double-blind, placebo-controlled trial". The Lancet Neurology. 14 (1): 39–47. doi:10.1016/S1474-4422(14)70262-5. PMID 25467848.
{{cite journal}}: CS1 maint: numeric names: authors list (link)
