Cyclic peptide
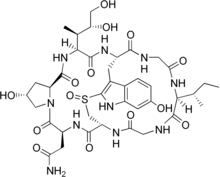


Cyclic peptides are polypeptide chains which contain a circular sequence of bonds.[1] This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in alpha-amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents.[2] Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.[3]
Classification
[edit]Cyclic peptides can be classified according to the types of bonds that comprise the ring.
- Homodetic cyclic peptides, such as cyclosporine A, are those in which the ring is composed exclusively of normal peptide bonds (i.e. between the alpha carboxyl of one residue to the alpha amine of another). The smallest such species are 2,5-diketopiperazines,[4] being derived from the cyclisation of a dipeptide.
- Cyclic isopeptides contain at least one non-alpha amide linkage, such as a linkage between the side chain of one residue to the alpha carboxyl group of another residue, as in microcystin and bacitracin.
- Cyclic depsipeptides, such as aureobasidin A and HUN-7293, have at least one lactone (ester) linkage in place of one of the amides. Some cyclic depsipeptides are cyclized between the C-terminal carboxyl and the side chain of a Thr or Ser residue in the chain, such as kahalalide F, theonellapeptolide, and didemnin B.
- Bicyclics such as the amanitins and the phalloidins contain a bridging group, generally between two of the side chains. In the amatoxins, this is formed as a sulfoxide bridge between the Trp and Cys residues. Other bicyclic peptides include echinomycin, triostin A, and Celogentin C.
- There are a number of bi and monocyclic peptides which are cyclized through a disulfide bond between two cysteines, oxytocin being a notable example.
- Cyclotides are a family of cysteine-rich peptides characterized by six conserved cysteines that form three disulfide bonds within the cyclic structure forming the characteristic cyclic cystine knot (CCK) that confers these peptides their characteristic 3D structure and it is believed to be greatly responsible for the stability and properties of the cyclotides.[5]
Biosynthesis
[edit]Cyclic peptides in plants are synthesized via a two-step process; the translation of a linear peptide chain, and its subsequent formation into a cyclic structure through activities of a protease-like enzyme or other ways.[6][7][8]
Some peptides, such as cyclotides, are gene-coded products obtained by the processing of larger precursor proteins. The generic configuration of the precursor protein consists of an endoplasmic reticulum signal sequence, a non-conserved pro-region, a highly conserved region known as the N-terminal repeat (NTR), the mature cyclotide domain and finally a short hydrophobic C-terminal tail.[9][10]
Properties and applications
[edit]Cyclic peptides tend to be extremely resistant to the process of digestion, making them of interest to scientists working on novel oral medications.[11]
Examples include:
See also
[edit]- Nonribosomal peptide
- lantibiotics, 19-37 residues and 1 to 5 'bridges'
References
[edit]- ^ Salehi, David; Mozaffari, Saghar; Zoghebi, Khalid; Lohan, Sandeep; Mandal, Dindyal; Tiwari, Rakesh K.; Parang, Keykavous (2022-03-29). "Amphiphilic Cell-Penetrating Peptides Containing Natural and Unnatural Amino Acids as Drug Delivery Agents". Cells. 11 (7): 1156. doi:10.3390/cells11071156. ISSN 2073-4409. PMC 8997995. PMID 35406720.
- ^ Jensen, Knud (2009-09-01). Peptide and Protein Design for Biopharmaceutical Applications. John Wiley & Sons. ISBN 9780470749715.
- ^ Wenyan, Xu; Jun, Tang; Changjiu, Ji; Wenjun, He; Ninghua, Tan (2008). "Application of a TLC chemical method to detection of cyclotides in plants". Science Bulletin. 53 (11): 1671–1674. Bibcode:2008SciBu..53.1671W. doi:10.1007/s11434-008-0178-8.
- ^ Borthwick AD (May 2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ^ de Veer, Simon J.; Kan, Meng-Wei; Craik, David J. (2019-12-26). "Cyclotides: From Structure to Function". Chemical Reviews. 119 (24): 12375–12421. doi:10.1021/acs.chemrev.9b00402. ISSN 0009-2665. PMID 31829013.
- ^ Barber, Carla J. S.; Pujara, Pareshkumar T.; Reed, Darwin W.; Chiwocha, Shiela; Zhang, Haixia; Covello, Patrick S. (2013). "The Two-step Biosynthesis of Cyclic Peptides from Linear Precursors in a Member of the Plant Family Caryophyllaceae Involves Cyclization by a Serine Protease-like Enzyme". Journal of Biological Chemistry. 288 (18): 12500–12510. doi:10.1074/jbc.M112.437947. PMC 3642298. PMID 23486480.
- ^ Wenyan Xu; et al. (2011). "Various mechanisms in cyclopeptide production from precursors synthesized independently of non-ribosomal peptide synthetases". Acta Biochimica et Biophysica Sinica. 43 (10): 757–762. doi:10.1093/abbs/gmr062. PMC 3180235. PMID 21764803.
- ^ Wenyan Xu; et al. "Plant Cyclopeptides and Possible Biosynthetic Mechanisms".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Dutton, Julie L.; Renda, Rosemary F.; Waine, Clement; Clark, Richard J.; Daly, Norelle L.; Jennings, Cameron V.; Anderson, Marilyn A.; Craik, David J. (November 2004). "Conserved Structural and Sequence Elements Implicated in the Processing of Gene-encoded Circular Proteins". Journal of Biological Chemistry. 279 (45): 46858–46867. doi:10.1074/jbc.M407421200. PMID 15328347.
- ^ Shafee, Thomas; Harris, Karen; Anderson, Marilyn (2015), "Biosynthesis of Cyclotides", Advances in Botanical Research, vol. 76, Elsevier, pp. 227–269, doi:10.1016/bs.abr.2015.08.005, ISBN 978-0-12-800030-4, retrieved 2024-09-25
- ^ David J. Craik (17 March 2006). "Seamless Proteins Tie Up Their Loose Ends". Science. 311 (5767): 1563–7. doi:10.1126/science.1125248. PMID 16543448. S2CID 82425866.
External links
[edit]- Cybase
- Cyclic+Peptides at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
