Peroxydisulfate

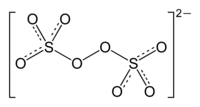
The peroxydisulfate ion, S
2O2−
8, is an oxyanion. It is commonly referred to as the persulfate ion or peroxodisulfate anions,[1] but this term also refers to the peroxomonosulfate ion, SO2−
5.In this oxidation states of both sulphur's is +6 ,also known as marshals acid. Approximately 500,000 tons of salts containing this anion are produced annually. Important salts include sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), and ammonium persulfate ((NH4)2S2O8). These salts are colourless, water-soluble solids that are strong oxidants.
Applications
Salts of peroxydisulfate are mainly used to initiate the polymerization of various alkenes, including styrene, acrylonitrile, and fluoroalkenes. Polymerization is initiated by the homolysis of the peroxydisulfate:
- [O3SO–OSO3]2− ⇌ 2 [SO4]•−
Moreover, sodium peroxydisulfate can be used for soil and groundwater remediation, water and wastewater treatment, and etching of copper on circuit boards.[2][1]
It has also been used to produce hair lighteners and bleaches, medical drugs, cellophane, rubber, soaps, detergents, adhesive papers, dyes for textiles, and in photography.[1]
In addition to its major commercial applications, peroxydisulfate participates in reactions of interest in the laboratory:
- Elbs persulfate oxidation
- Oxidation of Ag+ to Ag2+
References
- ^ a b c Shafiee, Saiful Arifin; Aarons, Jolyon; Hamzah, Hairul Hisham (2018). "Electroreduction of Peroxodisulfate: A Review of a Complicated Reaction". Journal of the Electrochemical Society. 165 (13). ECS: H785–H798. doi:10.1149/2.1161811jes.
- ^ Wacławek, S., Lutze, H. V., Grübel, K., Padil, V.V.T., Černík, M., Dionysiou, D.D. (2017) (2017). "Chemistry of persulfates in water and wastewater treatment: A review". Chemical Engineering Journal. 330: 44–62. doi:10.1016/j.cej.2017.07.132.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link)
