Phosphaalkyne
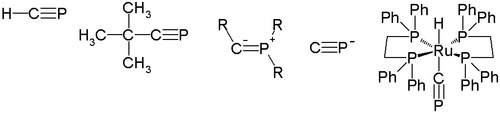
In chemistry, phosphaalkyne is a molecule which has a phosphorus-carbon triple bond [1]. This type of compound is widely studied in organic chemistry but does not exist outside the laboratory.
There are two types of phosphaalkynes. One type of phosphaalkyne is a heavier analogue of nitriles (R-C≡N). Another type of phosphaalkyne has a five-valent three coordinate phosphorus. This molecule can also be described as ylide or a phosphinocarbene. The phosphorus pendant of the isonitriles R-P+≡C- called isophosphaalkynes has never been observed.
In 1950, H. Albers reported the first indication of the existence of the parent compound of phosphaalkynes (type A), H-C≡P. This compound was identified by infrared absorption spectrometry and its synthesis was improved by Manfred Regitz in 1987. The synthesis of the first kinetically stable phosphaalkyne, which has a tert-butyl group as a substituent R, was reported in 1981 by Gerd Becker and Werner Uhl. These phosphaalkynes exhibit 1,2-addition reactions and cycloadditions in their reactivity.
In 2000, Guy Bertrand reported the first structure of the type B phosphaalkyne. Its P-C-R bond angle is 152.6 degrees, so this type of phosphaalkyne may be best described by a phosphorus vinyl ylide structure (B2).
The cyaphide ion P≡C− as the phosphorus cyanide cousin is not known as a salt and only observed in the gas phase. In silico measurements reveal that the -1 charge in this ion is location mainly on carbon (0.65). On the other hand the molecule does exist as a terminal ligand in a certain ruthenium transition metal complex trans-[(dppe)2Ru-(H)(C≡P)] stabilized by dppe [2].
References
- ^ Cyaphide (C≡P-): The Phosphorus Analogue of Cyanide (C≡N-)Robert J. Angelici Angew. Chem. Int. Ed. 2007, 46, 330 – 332 doi:10.1002/anie.200603724
- ^ Making the True "CP" Ligand. Cordaro et al. Angew. Chem. Int. Ed. 2006, 45, 6159 - 6162 doi:10.1002/anie.200602499
