Phosphorylation
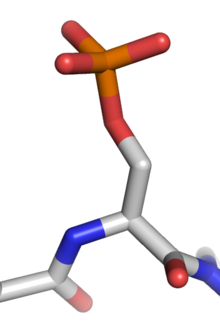
Phosphorylation is the addition of a phosphate (PO43-) group to a protein or other organic molecule. Phosphorylation turns many protein enzymes on and off, thereby altering their function and activity.
Protein phosphorylation in particular plays a significant role in a wide range of cellular processes. Its prominent role in biochemistry is the subject of a very large body of research (as of March 2012, the Medline database returns nearly 200,000 articles on the subject, largely on protein phosphorylation).
Protein phosphorylation
History
In 1906, Phoebus A. Levene at the Rockefeller Institute for Medical Research identified phosphate in the protein vitellin (phosvitin),[1] and by 1933 had detected phosphoserine in casein, with Fritz Lipmann.[2] However, it took another 20 years before Eugene P. Kennedy described the first ‘enzymatic phosphorylation of proteins’.[3]
Function
Reversible phosphorylation of proteins is an important regulatory mechanism that occurs in both prokaryotic and eukaryotic organisms.[4][5][6][7] Kinases phosphorylate proteins and phosphatases dephosphorylate proteins. Many enzymes and receptors are switched "on" or "off" by phosphorylation and dephosphorylation. Reversible phosphorylation results in a conformational change in the structure in many enzymes and receptors, causing them to become activated or deactivated. Phosphorylation usually occurs on serine, threonine, tyrosine and histidine residues in eukaryotic proteins. Histidine phosphorylation of eukaryotic proteins appears to be much more frequent than tyrosine phosphorylation.[8] In prokaryotic proteins phosphorylation occurs on the serine, threonine, tyrosine, histidine or arginine or lysine residues.[4][5][8][9] The addition of a phosphate (PO4) molecule to a polar R group of an amino acid residue can turn a hydrophobic portion of a protein into a polar and extremely hydrophilic portion of molecule. In this way it can introduce a conformational change in the structure of the protein via interaction with other hydrophobic and hydrophilic residues in the protein.
One such example of the regulatory role that phosphorylation plays is the p53 tumor suppressor protein. The p53 protein is heavily regulated[10] and contains more than 18 different phosphorylation sites. Activation of p53 can lead to cell cycle arrest, which can be reversed under some circumstances, or apoptotic cell death.[11] This activity occurs only in situations wherein the cell is damaged or physiology is disturbed in normal healthy individuals.
Upon the deactivating signal, the protein becomes dephosphorylated again and stops working. This is the mechanism in many forms of signal transduction, for example the way in which incoming light is processed in the light-sensitive cells of the retina.
Regulatory roles of phosphorylation include
- Biological thermodynamics of energy-requiring reactions
- Phosphorylation of Na+/K+-ATPase during the transport of sodium (Na+) and potassium (K+) ions across the cell membrane in osmoregulation to maintain homeostasis of the body's water content.
- Mediates enzyme inhibition
- Phosphorylation of the enzyme GSK-3 by AKT (Protein kinase B) as part of the insulin signaling pathway.[12]
- Phosphorylation of src tyrosine kinase (pronounced "sarc") by C-terminal Src kinase (Csk) induces a conformational change in the enzyme, resulting in a fold in the structure, which masks its kinase domain, and is thus shut "off".[13]
- Important for protein-protein interaction via "recognition domains."
- Phosphorylation of the cytosolic components of NADPH oxidase, a large membrane-bound, multi-protein enzyme present in phagocytic cells, plays an important role in the regulation of protein-protein interactions in the enzyme.[14]
- Important in protein degradation.
- In the late 1990s, it was recognized that phosphorylation of some proteins causes them to be degraded by the ATP-dependent ubiquitin/proteasome pathway. These target proteins become substrates for particular E3 ubiquitin ligases only when they are phosphorylated.
Signaling networks
Elucidating complex signaling pathway phosphorylation events can be difficult. In a cellular signaling pathways, a protein A phosphorylates protein B, and B phosphorylates C. However, in another signaling pathway, protein D phosphorylates A, or phosphorylates protein C. Global approaches such as phosphoproteomics, the study of phosphorylated proteins, which is a sub-branch of proteomics, combined with mass spectrometry-based proteomics, have been utilised to identify and quantify dynamic changes in phosphorylated proteins over time. These techniques are becoming increasingly important for the systematic analysis of complex phosphorylation networks.[15] They have been successfully used to identify dynamic changes in the phosphorylation status of more than 6000 sites after stimulation with epidermal growth factor.[16] Another approach for understanding Phosphorylation Network, is by measuring the genetic interactions between multiple phosphorylating proteins and their targets. This reveals interesting recurring patterns of interactions - network motifs.[17]
Protein phosphorylation sites
There are thousands of distinct phosphorylation sites in a given cell since: 1) There are thousands of different kinds of proteins in any particular cell (such as a lymphocyte). 2) It is estimated that 1/10 to 1/2 of proteins are phosphorylated (in some cellular state). 3) Phosphorylation often occurs on multiple distinct sites on a given protein.
Since phosphorylation of any site on a given protein can change the function or localization of that protein, understanding the "state" of a cell requires knowing the phosphorylation state of its proteins. For example, if amino acid Serine-473 ("S473") in the protein AKT is phosphorylated, AKT is, in general, functionally active as a kinase. If not, it is an inactive kinase.
Types of phosphorylation
See also kinases for more details on the different types of phosphorylation
Within a protein, phosphorylation can occur on several amino acids. Phosphorylation on serine is the most common, followed by threonine. Tyrosine phosphorylation is relatively rare. However, since tyrosine phosphorylated proteins are relatively easy to purify using antibodies, tyrosine phosphorylation sites are relatively well understood. Histidine and aspartate phosphorylation occurs in prokaryotes as part of two-component signaling and in some cases in eukaryotes in some signal transduction pathways.[18]
Detection and characterization
Antibodies can be used as powerful tools to detect whether a protein is phosphorylated at a particular site. Antibodies bind to and detect phosphorylation-induced conformational changes in the protein. Such antibodies are called phospho-specific antibodies; hundreds of such antibodies are now available.[19] They are becoming critical reagents both for basic research and for clinical diagnosis.
PTM (Posttranslational Modification) isoforms are easily detected on 2D gels. Indeed, phosphorylation replaces neutral hydroxyl groups on serines, threonines, or tyrosines with negatively-charged phosphates with pKs near 1.2 and 6.5. Thus, below pH 5.5, phosphates add a single negative charge; near pH 6.5, they add 1.5 negative charges; above pH 7.5, they add 2 negative charges. The relative amount of each isoform can also easily and rapidly be determined from staining intensity on 2D gels.
In some very specific cases, the detection of the phosphorylation as a shift in the protein's electrophoretic mobility is possible on simple 1-dimensional SDS-PAGE gels, as it's described for instance for a transcriptional coactivator by Kovacs et al.[20] Strong phosphorylation-related conformational changes (that persist in detergent-containing solutions) are thought to underlie this phenomenon. Most of the phosphorylation sites for which such a mobility shift has been described fall in the category of SP and TP sites (i.e. a proline residue follows the phosphorylated serine or threonine residue).
More recently large-scale mass spectrometry analyses have been used to determine sites of protein phosphorylation. Over the last 4 years, dozens of studies have been published, each identifying thousands of sites, many of which were previously undescribed.[21][22] Mass spectrometry is ideally suited for such analyses, as the addition of phosphorylation results in an increase in the mass of the protein and the phosphorylated residue. However, advanced, highly accurate mass spectrometers are needed for these studies, limiting the technology to labs with high-end mass spectrometers.
A detailed characterization of the sites of phosphorylation is very difficult, and the quantitation of protein phosphorylation by mass spectrometry requires isotopic internal standard approaches.[23] A relative quantitation can be obtained with a variety of differential isotope labeling technologies.[24] There are also several quantitative protein phosphorylation methods, including fluorescence immunoassays, Microscale Thermophoresis, FRET, TRF, fluorescence polarization, fluorescence-quenching, mobility shift, bead-based detection, and cell-based formats.[25][26]
Other kinds
ATP, the "high-energy" exchange medium in the cell, is synthesized in the mitochondrion by addition of a third phosphate group to ADP in a process referred to as oxidative phosphorylation. ATP is also synthesized by substrate-level phosphorylation during glycolysis. ATP is synthesized at the expense of solar energy by photophosphorylation in the chloroplasts of plant cells.
Phosphorylation of sugars is often the first stage of their catabolism. It allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter.
See also
References
- ^ Levene PA; Alsberg CL (1906). "The cleavage products of vitellin" (PDF). J. Biol. Chem. 2 (1): 127–133.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|author-separator=ignored (help) - ^ Lipmann FA; Levene PA (1932). "Serinephosphoric acid obtained on hydrolysis of vitellinic acid" (PDF). J. Biol. Chem. 98 (1): 109–114.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Burnett G; Kennedy EP (1954). "The enzymatic phosphorylation of proteins" (PDF). J. Biol. Chem. 211 (2): 969–80. PMID 13221602.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b Cozzone AJ (1988). "Protein phosphorylation in prokaryotes". Annu. Rev. Microbiol. 42: 97–125. doi:10.1146/annurev.mi.42.100188.000525. PMID 2849375.
- ^ a b Stock JB; Ninfa AJ; Stock AM (1989). "Protein phosphorylation and regulation of adaptive responses in bacteria". Microbiol. Rev. 53 (4): 450–90. PMC 372749. PMID 2556636.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Chang C; Stewart RC (1998). "The Two-Component System . Regulation of Diverse Signaling Pathways in Prokaryotes and Eukaryotes". Plant Physiol. 117 (3): 723–31. doi:10.1104/PPSOE.117.3.723. PMC 1539182. PMID 9662515.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Barford D; Das AK; Egloff MP (1998). "The structure and mechanism of protein phosphatases: insights into catalysis and regulation". Annu Rev Biophys Biomol Struct. 27: 133–64. doi:10.1146/annurev.biophys.27.1.133. PMID 9646865.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ a b Ciesla J; Fraczyk T; Rode W (2011). "Phosphorylation of basic amino acid residues in proteins: important but easily missed". Acta Biochim Pol. 58 (2): 137–47. PMID 21623415.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Deutscher J; Saier (2005). "Ser/Thr/Tyr protein phosphorylation in bacteria - for long time neglected, now well established". J Mol Microbiol Biotechnol. 9 (3–4): 125–31. doi:10.1159/000089641. PMID 16415586.
{{cite journal}}: More than one of|author2=and|last2=specified (help); Unknown parameter|author-separator=ignored (help) - ^ Ashcroft M; Kubbutat MH; Vousden KH (1999). "Regulation of p53 Function and Stability by Phosphorylation". Mol. Cell. Biol. 19 (3): 1751–8. PMC 83968. PMID 10022862.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Bates S; Vousden KH (1996). "p53 in signaling checkpoint arrest or apoptosis". Curr. Opin. Genet. Dev. 6 (1): 12–8. doi:10.1016/S0959-437X(96)90004-0. PMID 8791489.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ van Weeren PC; de Bruyn KM; de Vries-Smits AM; van Lint J; Burgering BM (1998). "Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB". J. Biol. Chem. 273 (21): 13150–6. doi:10.1074/jbc.273.21.13150. PMID 9582355.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Cole PA; Shen K; Qiao Y; Wang D (2003). "Protein tyrosine kinases Src and Csk: a tail's tale". Curr Opin Chem Biol. 7 (5): 580–5. doi:10.1016/j.cbpa.2003.08.009. PMID 14580561.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Babior BM (1999). "NADPH oxidase: an update". Blood. 93 (5): 1464–76. PMID 10029572.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Olsen JV; Blagoev B; Gnad F; Macek B; Kumar C; Mortensen P; Mann M (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Li-Rong Y; Issaq HJ; Veenstra TD (2007). "Phosphoproteomics for the discovery of kinases as cancer biomarkers and drug targets". Proteomics - Clinical Applications. 1 (9): 1042–1057. doi:10.1002/prca.200700102. PMID 21136756.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|author-separator=ignored (help) - ^ Fiedler D; Braberg H; Mehta M; Chechik G; Cagney, Gerard; Mukherjee, Paromita; Silva, Andrea C.; Shales, Michael; Collins, Sean R. (2009). "Functional Organization of the S. cerevisiae Phosphorylation Network". Cell. 136 (5): 952–963. doi:10.1016/j.cell.2008.12.039. PMC 2856666. PMID 19269370.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|author-separator=ignored (help) - ^ Thomason P; Kay R (2000). "Eukaryotic signal transduction via histidine-aspartate phosphorelay" (PDF). J. Cell. Sci. 113 (18): 3141–50. PMID 10954413.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ "phospho antibody". exactantigen.com. Retrieved 2009-01-22.
- ^ Kovacs KA, Steinmann M (2003). "CCAAT/enhancer-binding protein family members recruit the coactivator CREB-binding protein and trigger its phosphorylation". J Biol. Chem. 278 (38). UNITED STATES: 36959–65. doi:10.1074/jbc.M303147200. ISSN 0021-9258. PMID 12857754.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Munton RP; Tweedie-Cullen R; Livingstone-Zatchej M; Weinandy F; Waidelich M; Longo D; Gehrig P; Potthast F; Rutishauser D (2007). "Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations". Mol. Cell Proteomics. 6 (2): 283–93. doi:10.1074/mcp.M600046-MCP200. PMID 17114649.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Trinidad JC; Thalhammer A; Specht CG; Lynn AJ; Baker PR; Schoepfer R; Burlingame AL (2008). "Quantitative analysis of synaptic phosphorylation and protein expression". Mol. Cell Proteomics. 7 (4): 684–96. doi:10.1074/mcp.M700170-MCP200. PMID 18056256.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Gerber SA; Rush J; Stemman O; Kirschner MW; Gygi SP (2003). "Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS". Proc. Natl. Acad. Sci. U.S.A. 100 (12): 6940–5. doi:10.1073/pnas.0832254100. PMC 165809. PMID 12771378.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Gygi SP; Rist B; Griffin TJ; Eng J; Aebersold R (2002). "Proteome analysis of low-abundance proteins using multidimensional chromatography and isotope-coded affinity tags". J. Proteome Res. 1 (1): 47–54. doi:10.1021/pr015509n. PMID 12643526.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Olive DM (2004). "Quantitative methods for the analysis of protein phosphorylation in drug development". Expert Rev Proteomics. 1 (3): 327–41. doi:10.1586/14789450.1.3.327. PMID 15966829.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chen H; Kovar J; Sissons S; Cox K; Matter W; Chadwell F; Luan P; Vlahos CJ; Schutz-Geschwender A (2005). "A cell-based immunocytockemical assay for monitoring kinase signaling pathways and drug efficacy". Anal. Biochem. 338 (1): 136–42. doi:10.1016/j.ab.2004.11.015. PMID 15707944.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help)
