Rucaparib
| |
| Names | |
|---|---|
| IUPAC name
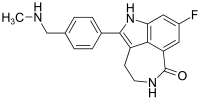
8-Fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one
| |
| Other names
AG014699
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.247.490 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18FN3O | |
| Molar mass | 323.371 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rucaparib (AG 014699) is a PARP inhibitor being investigated as a potential anti-cancer agent. Rucaparib is the first-in-class clinical candidate targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1), and was first synthesised as part of a collaboration between scientists working in Northern Institute of Cancer Research and Medical School of Newcastle University, alongside Agouron Pharmaceuticals (San Diego).[1] It is being developed by Clovis Oncology.
It can be taken orally in tablet form.[2] It is a benzimidazole derivative (being a 1H-benzimidazole-4-carboxamide).[1]
Biological effects
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture."[3]
Mechanism of action
As a PARP inhibitor it is expected to be more effective in cancers with a BRCA mutation (BRCA1 or BRCA2). e.g. about nine percent of pancreatic patients are BRCA1/BRCA2 positive.[4]
Clinical trials
It has undergone phase I clinical trials for patients with advanced solid tumours.[5] It is in phase II clinical trials for metastatic breast and ovarian cancer with known BRCA1 or BRCA2 mutation.[2][6]
It is thought that 20% of women with ovarian cancer who are not BRCA positive might also benefit from PARP inhibitors.[citation needed]
As of April 2016[update] the ARIEL3 phase III clinical trial for maintenance after platinum-based chemotherapy for serous and endometrioid ovarian cancer is active.[7]
As of June 2016[update] six clinical trials of rucaparib were active.[8]
June 2016 some encouraging results from a small early trial on BRCA1/2 positive pancreatic cancer were announced.[4]
References
- ^ a b "Resistance-Modifying Agents. 9.1 Synthesis and Biological Properties of Benzimidazole Inhibitors of the DNA Repair Enzyme Poly(ADP-ribose) Polymerase". J Med Chem. 2000 Nov 2;43(22):4084-97. doi:10.1021/jm000950v.
- ^ a b "Cancer Research launches new drug trial". 11 Jan 2011.
- ^ http://www.qub.ac.uk/schools/SchoolofPharmacy/Filestore/Filetoupload,121186,en.pdf
- ^ a b Rucaparib shows clinical benefit in pancreatic cancer patients with BRCA mutation. June 2016
- ^ "First in human phase I trial of the PARP inhibitor AG-014699 with temozolomide (TMZ) in patients (pts) with advanced solid tumors".
- ^ http://science.cancerresearchuk.org/research/loc/newcastle/newcastle_univ/plummerr/plummerrfr/plummerrfrp2/?version=1 URL no longer relevant
- ^ A Study of Rucaparib as Switch Maintenance Following Platinum-Based Chemotherapy in Patients With Platinum-Sensitive, High-Grade Serous or Endometrioid Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer (ARIEL3)
- ^ Rucaparib trials
