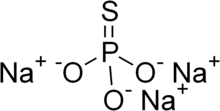
Sodium monothiophosphate
| |
| Names | |
|---|---|
| IUPAC name
Sodium monothiophosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.030.224 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na3PO3S | |
| Molar mass | 180.030 g/mol |
| Appearance | Crystalline white solid |
| Melting point | 120 to 125 °C (248 to 257 °F; 393 to 398 K) (decomposition) |
| Miscible | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium monothiophosphate, or sodium phosphorothioate, is a chemical compound with the molecular formula Na3PO3S. It is a crystalline white solid that decomposes without melting at 120-125 °C.
Preparation
Sodium monothiophosphate is created via the reaction between thiophosphoryl chloride and sodium hydroxide according to the method of Yasuda and Lambert.[1]
- PSCl3 + 6 NaOH → Na3PO3S + 3 NaCl + 3 H2O
The yield depends on the purity of the sodium hydroxide. Also, sodium phosphorothiolate decomposes at neutral pH and so it was found that using an excess of sodium hydroxide will allow for a greater yield since the reaction will not near a neutral pH. Also, it was noted that silicone grease catalyses the hydrolysis of the phosphorothioate ion, so it is recommended that it is not used in the glass joints.[2] The reported average yield was 59% and it is possible to get higher if the pH is altered.[3]
References
- ^ S. K. Yasuda and J. L. Lambert, Inorganic Syntheses, 5, 102 (1957).
- ^ Lucian C. Pop and M. Saito (2015). "Serendipitous Reactions Involving a Silicone Grease". Coordination Chemistry Reviews. doi:10.1016/j.ccr.2015.07.005.
- ^ L. C. Washburn and R. L. Hayes, Inorganic Syntheses, 17, 193
