Sodium sorbate
Appearance
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
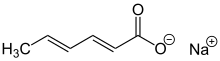
sodium (2E,4E)-hexa-2,4-dienoate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.927 | ||
| E number | E201 (preservatives) | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H7NaO2 | |||
| Molar mass | 134.10835 g/mol | ||
| Odor | hydrocarbon-like | ||
| Boiling point | 233 °C (451 °F; 506 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sodium sorbate is the sodium salt of sorbic acid. Its formula is NaC6H7O2 and systematic name is sodium (E,E)-hexa-2,4-dienoate.
It is a food additive with E-number E201.
Safety and health effects
Unlike other sorbic acid salts such as potassium sorbate (E202) and calcium sorbate (E203), the use of sodium sorbate as a food additive is not allowed in the EU due to potential genotoxic effects.[2]
References


