From Wikipedia, the free encyclopedia
Sovaprevir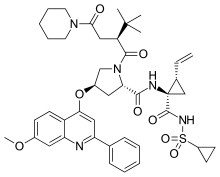 |
|
| Other names | ACH-1625 |
|---|
|
| Legal status |
|
|---|
|
(4R)-N-{(1R,2S)-1-[(Cyclopropylsulfonyl)carbamoyl]-2-vinylcyclopropyl}-1-{(2S)-3,3-dimethyl-2-[2-oxo-2-(1-piperidinyl)ethyl]butanoyl}-4-[(7-methoxy-2-phenyl-4-quinolinyl)oxy]-L-prolinamide
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C43H53N5O8S |
|---|
| Molar mass | 799.98 g/mol g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
CC(C)(C)[C@H](CC(=O)N1CCCCC1)C(=O)N2C[C@@H](C[C@H]2C(=O)N[C@@]3(C[C@H]3C=C)C(=O)NS(=O)(=O)C4CC4)Oc5cc(nc6c5ccc(c6)OC)c7ccccc7
|
InChI=1S/C43H53N5O8S/c1-6-28-25-43(28,41(52)46-57(53,54)31-16-17-31)45-39(50)36-22-30(26-48(36)40(51)33(42(2,3)4)23-38(49)47-19-11-8-12-20-47)56-37-24-34(27-13-9-7-10-14-27)44-35-21-29(55-5)15-18-32(35)37/h6-7,9-10,13-15,18,21,24,28,30-31,33,36H,1,8,11-12,16-17,19-20,22-23,25-26H2,2-5H3,(H,45,50)(H,46,52)/t28-,30-,33-,36+,43-/m1/s1 Key:MHFMTUBUVQZIRE-WINRQGAFSA-N
|
Sovaprevir (ACH-1625) is an experimental drug designed to treat the hepatitis C virus. It is under development by Achillion Pharmaceuticals.
Sovaprevir received Fast Track status from the U.S. Food and Drug Administration (FDA) in 2012.[1]
References
External links

