Strecker amino acid synthesis
The Strecker amino acid synthesis, devised by Adolph Strecker, is a series of chemical reactions that synthesize an amino acid from an aldehyde (or ketone).[1][2] The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino-acid. [3] [4]
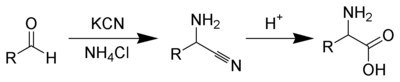
While usage of ammonium salts gives unsubstituted amino acids, primary and secondary amines also successfully give substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids. [5]
The traditional synthesis of Adolph Strecker from 1850 gives racemic α-amino nitriles, but recently several procedures utilizing asymmetric auxiliaries [6] or asymmetric catalysts [7] [8] have been developed. [9]
Reaction mechanism
The reaction mechanism for this reaction is sketched below. In part one aldehyde 1.1 reacts with ammonia in a nucleophilic addition to the hemiaminal 1.3 which attracts a proton to form iminium ion 1.5 by elimination of water. A second nucleophilic addition of the cyanide ion forms the aminonitrile 1.6.

|

| |
| Strecker synthesis part I | Strecker synthesis part II |
In stage two a proton activates aminonitrile 2.1 for nucleophilic addition of two equivalents of water to intermediate 2.6 which eliminates ammonia and a proton to final product 2.7.
Scope
An example of present-day use of the Strecker synthesis is a multikilogram scale synthesis of a L-valine derivative starting from 3-methyl-2-butanone [10] [11]:
References
- ^ Strecker, A. (1850). "Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper". Annalen der Chemie und Pharmazie. 75 (1): 27–45. doi:10.1002/jlac.18500750103.
- ^ Strecker, A. (1854). "Ueber einen neuen aus Aldehyd - Ammoniak und Blausäure entstehenden Körper (p )". Annalen der Chemie und Pharmazie. 91 (3): 349–351. doi:10.1002/jlac.18540910309.
- ^ Kendall, E. C.; McKenzie, B. F. Organic Syntheses, Coll. Vol. 1, p.21 (1941); Vol. 9, p.4 (1929). (Article)
- ^ Clarke, H. T.; Bean, H. J. Organic Syntheses, Coll. Vol. 2, p.29 (1943); Vol. 11, p.4 (1931). (Article)
- ^ Masumoto, S.; Usuda, H.; Suzuki, M.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2003, 125(19), 5634-5635. (doi:10.1021/ja034980+)
- ^ Davis, F. A. et al. Tetrahedron Lett. 1994, 35, 9351.
- ^ Ishitani, H.; Komiyama, S.; Hasegawa, Y.; Kobayashi, S. J. Am. Chem. Soc. 2000, 122(5), 762-766. (doi:10.1021/ja9935207)
- ^ Huang, J.; Corey, E. J. Org. Lett. 2004, 6(26), 5027-5029. (doi:10.1021/ol047698w)
- ^ Duthaler, R. O. Tetrahedron 1994, 50, 1539-1650. (Review, doi:10.1016/S0040-4020(01)80840-1)
- ^ A Concise Synthesis of (S)-N-Ethoxycarbonyl--methylvaline Jeffrey T. Kuethe, Donald R. Gauthier, Jr., Gregory L. Beutner, and Nobuyoshi Yasuda J. Org. Chem., 72 (19), 7469 -7472, 2007. doi:10.1021/jo7012862
- ^ The initial reaction product of 3-methyl-2butanone with sodium cyanide and ammonia is resolved by application of L-tartaric acid. The amino acid is isolated as its salt with dicyclohexylamine.

