Thioacetal
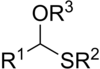

Thioacetals are the sulfur analogues of acetals. There are two classes: monothioacetals and dithioacetals. Monothioacetals are less common, have the functional group RC(OR')(SR")H. Dithioacetals have the formula RC(SR')2H (symmetric dithioacetals) and RC(SR')(SR")H (asymmetric dithioacetals).[1]
The symmetric dithioacetals are relativesly common. They are prepared by condensation of thiols or dithiols with aldedhydes. These reactions proceed via the intermediacy of hemithioacetals:
- thiol addition to give hemithioacetal: RSH + R'CH(O) → R'CH(SR)(OH)
- thiol addition with loss of water, affording dithioacetal: RSH + R'CH(OH)SR → R'CH(SR)2 + H2O
Such reactions typically employ either a Lewis or Brønsted acid catalyst.
Dithioacetals generated from aldehydes and 1,2-ethanedithiol and 1,3-propanedithiol are useful in organic synthesis:[2]
- C2H4(SH)2 + R'CHO → R'CHS2C2H4 + H2O
While the carbonyl carbon of an aldehyde is electrophilic, the deprotonated derivatives of dithioacetals feature nucleophilic carbon centers:
- R'CHS2C2H4 + R2NLi → R'CLiS2C2H4 + R2NH
Again, this reactivity is most commonly exploited in the 1,3-dithiolanes. The inversion of polarity between R'(H)Cδ+Oδ− and R'CLi(SR)2 is referred to as umpolung.
References
- ^ http://goldbook.iupac.org/T06348.html
- ^ P. Stütz And P. A. Stadler "3-alkylated And 3-acylated Indoles From A Common Precursor: 3-benzylindole And 3-benzoylindole" Org. Synth. 1977, 56, 8.doi:10.15227/orgsyn.056.0008
