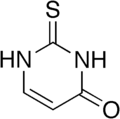
2-Thiouracil
Appearance
| |
| Names | |
|---|---|
| IUPAC name
2-Thioxo-1H-pyrimidin-4-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.008 |
| KEGG | |
| MeSH | Thiouracil |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H4N2OS | |
| Molar mass | 128.15 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiouracil refers both to a specific molecule consisting of a sulfated uracil, and a family of molecules based upon that structure.
Medical use
The substance is a historically relevant anti-thyroid preparation. Astwood E.B. used it in 1943 as therapy of Graves' disease for the first time.[1] It remains in use.
Thiouracil inhibits thyroid activity by blocking the enzyme thyroid peroxidase.[2] Its use in recent times has been replaced by advent of more potent and safer antithyroid drugs.
References
- ^ Gerabek, W. (2005). Enzyklopädie Medizingeschichte. p. 152. ISBN 9783110157147.
- ^ Nagasaka, A.; Hidaka, H. (1976). "Effect of Antithyroid Agents 6-Propyl-2-Thiouracil and l-Methyl-2-Mercaptoimidazole on Human Thyroid Iodide Peroxidase". Journal of Clinical Endocrinology & Metabolism. 43 (1): 152–8. doi:10.1210/jcem-43-1-152. PMID 947933.
