Trimethylenemethane
 Trimethylenemethane, average of three configurations. Formally, the radial bonds have valency 4/3. Each terminal carbon has 2/3 of an unfilled valence bond.
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylidenepropane-1,3-diyl | |
| Other names
Trimethylenemethane biradical; Trimethylenemethane diradical
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4H6 | |
| Molar mass | 54.092 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimethylenemethane (often abbreviated TMM) is a chemical compound with formula C
4H
6. It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene molecule C
4H
8 with two hydrogen atoms removed from the terminal methyl groups.
Structure
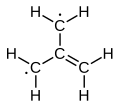
[edit]The electronic structure of trimethylenemethane was discussed in 1948.[1][2] It is a neutral four-carbon molecule containing four pi molecular orbitals. When trapped in a solid matrix at about 90 K (−183 °C), the six hydrogen atoms of the molecule are equivalent. Thus, it can be described either as zwitterion, or as the simplest conjugated hydrocarbon that cannot be given a Kekulé structure. It can be described as the superposition of three states:

|

|
|
It has a triplet ground state (3A2′/3B2), and is therefore a diradical in the stricter sense of the term.[3] Calculations predict a planar molecule with three-fold rotational symmetry, with approximate bond lengths 1.40 Å (C–C) and 1.08 Å (C–H). The H–C–H angle in each methylene is about 121°.[1]
Of the three singlet excited states, the first one, 11A1 (1.17 eV above ground), is a closed shell diradical with flat geometry and fully degenerate threefold (D3h) symmetry. The second one, 11B2 (also at 1.17 eV), is an open-shell radical with a D3h-symmetric equilibrium between three equal geometries; each has a longer C–C bond (1.48 Å) and two shorter ones (1.38 Å), and is flat and bilaterally symmetric except that the longer methylene is twisted 79° out of the plane (C2 symmetry). The third singlet state, 21A1/1A1′ (3.88 eV), is also a D3h-symmetric equilibrium of three geometries; each is planar with one shorter C–C bond and two longer ones (C2ν symmetry).[1]
The next higher energy states are degenerate triplets, 13A1 and 23B2 (4.61 eV), with one excited electron; and a quintet state, 5B2 (7.17 eV), with the p orbitals occupied by single electrons and D3h symmetry.[1]
Preparation
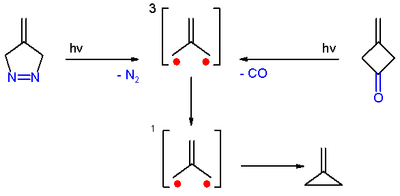
[edit]Trimethylenemethane was first obtained from photolysis of the diazo compound 4-methylene-Δ1-pyrazoline with expulsion of nitrogen, in a frozen dilute glassy solution at −196 °C (77 K).[3]
It was also obtained from photolysis of 3-methylenecyclobutanone, both in cold solution and in the form of a single crystal, with expulsion of carbon monoxide. In both cases, trimethylenemethane was detected by electron spin resonance spectroscopy.[3]
Trimethylenemethane has been obtained also by treating potassium with 2-iodomethyl-3-iodopropene and isobutylene diiodide (IH
2C)2C=CH
2 in the gas phase. However the product quickly dimerizes to yield 1,4-dimethylenecyclohexane, and also 2-methylpropene by abstracting two hydrogen atoms from other molecules (hydrocarbon or potassium hydride).[4]
Organometallic chemistry
[edit]A number of organometallic complexes have been prepared, starting with Fe(C
4H
6)(CO)3, which was obtained by the ring-opening of methylenecyclopropane with diiron nonacarbonyl (Fe
2(CO)9).[3] The same complex was prepared by the salt metathesis reaction of disodium tetracarbonylferrate (Na
2Fe(CO)4) with 1,1-bis(chloromethyl)ethylene (H2C=C(CH2Cl)2).[5] Related reactions give M(TMM)(CO)4 (M = Cr, Mo). The reaction leading to (TMM)Mo(CO)4 also gives Mo(C
8H
12)(CO)3 containing a dimerized TMM ligand.[5]
TMM complexes have been examine for their potential in organic synthesis, specifically in the trimethylenemethane cycloaddition reaction with only modest success. One example is a palladium-catalyzed [3+2]cycloaddition of trimethylenemethane.[6]
-
Structure of Ru(trimethylenemethane)(CO)3, viewed down C3 axis.[7]
-
Structure of Ru(trimethylenemethane)(CO)3, viewed orthogonal to C3 axis.
References
[edit]- ^ a b c d Slipchenko Lyudmila V., Krylov Anna I. (2003). "Electronic structure of the trimethylenemethane diradical in its ground and electronically excited states: Bonding, equilibrium geometries, and Vibrational frequencies". Journal of Chemical Physics. 118 (15): 6874–6883. Bibcode:2003JChPh.118.6874S. doi:10.1063/1.1561052. S2CID 4204676.
- ^ C. A. Coulson (1948), Journal de Chimie Physique et de Physico-Chimie Biologique, volume 45, page 243. Cited by Slipchenko and Krylov (2003)
- ^ a b c d Paul Dowd (1972). "Trimethylenemethane". Accounts of Chemical Research. 5 (7): 242–248doi=10.1021/ar50055a003. doi:10.1021/ar50055a003.
- ^ Skell Philip S., Doerr Robert G. (1967). "Trimethylenemethane". Journal of the American Chemical Society. 89 (18): 4688–4692. doi:10.1021/ja00994a020.
- ^ a b J. S. Ward & R. Pettit (1970). "Trimethylenemethane complexes of iron, molybdenum, and chromium". Journal of the Chemical Society D (21): 1419–1420. doi:10.1039/C29700001419.
- ^ Barry M. Trost (1979). "New conjunctive reagents. 2-Acetoxymethyl-3-allyltrimethylsilane for methylenecyclopentane annulations catalyzed by palladium(0)". Journal of the American Chemical Society. 101 (21): 6429–6432. doi:10.1021/ja00515a046.
- ^ Herberich, G. E.; Spaniol, T. P. (1993). "Trimethylenemethane Complexes of Ruthenium, Osmium and Rhodium Via the Compound CH2=C(CH2SnMe3)2". Journal of the Chemical Society, Dalton Transactions (16): 2471–2476. doi:10.1039/DT9930002471.

![Structure of Ru(trimethylenemethane)(CO)3, viewed down C3 axis.[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/82/PETCEWtopView.png/120px-PETCEWtopView.png)
