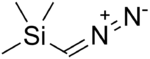
Trimethylsilyldiazomethane
| |
| Names | |
|---|---|
| IUPAC name
(diazomethyl)trimethylsilane
| |
| Other names
Trimethylsilyldiazomethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.131.243 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C4H10N2Si | |
| Molar mass | 114.223 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diazo(trimethylsilyl)methane, (CH3)3SiCHN2, is a diazo compound widely used as a less-explosive replacement for diazomethane.
Preparation
Diazo(trimethylsilyl)methane may be prepared from reacting (trimethylsilyl)methylmagnesium chloride with diphenyl phosphorazidate.[1]
Uses
Diazo(trimethylsilyl)methane is a commercially available reagent used in organic chemistry as a methylating agent. It is a less explosive alternative to diazomethane for the methylation of carboxylic acids. It also reacts with alcohols to give methyl ethers, where diazomethane may not.[2]
It has also been employed widely in tandem with GC-MS for the analysis of various carboxylic compounds which are ubiquitous in nature. The fact that the reaction is rapid and occurs readily makes it attractive. However, it can form artifacts which complicate spectral interpretation.
Safety
Inhalation of diazo(trimethylsilyl)methane is potentially fatal and has been implicated in the death of at least two chemists, a pharmaceutical worker in Windsor, Nova Scotia, Canada and one in New Jersey.[3][4][5]
References
- ^ Takayuki Shioiri, Toyohiko Aoyama, and Shigehiro Mori (1993). "Diazo(trimethylsilyl)methane". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 612. - ^ Armin Presser and Antje Hüfner (2004). "Diazo(trimethylsilyl)methane – A Mild and Efficient Reagent for the Methylation of Carboxylic Acids and Alcohols in Natural Products". Chemical Monthly. 135. doi:10.1007/s00706-004-0188-4.
- ^ Family says N.S. pharmaceutical worker named chemical he used before death, The Amherst Daily News, October 20, 2008
- ^ N.S. probe into pharma worker's death finds vent hoods turned off in lab, canadaeast.com, May 13th, 2009
- ^ Five safety charges laid in death of worker at N.S. drug factory,thechronicleherald.ca, April 14th, 2010
