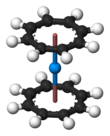
Uranocene
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
bis(η8-cyclooctatetraene) uranium
| |||
| Other names
uranium cyclooctatetraenyl, U(COT)2
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C16H16U | |||
| Molar mass | 446.33 g/mol | ||
| Appearance | green crystals[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
ignites in air | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Uranocene U(C8H8)2 is the most notable cyclooctatetraenide of the f elements and one of the first organouranium compounds to be synthesized. Uranocene is a member of the actinocenes, a group of metallocenes incorporating elements from the actinide series. It is the most studied bis[8]annulene-metal system.
Synthesis
Uranocene was first prepared by the reaction of uranium tetrachloride and dipotassium cyclooctatetraene, viz.
- 2 K + C8H8 → K2(C8H8)
- 2 K2(C8H8) + UCl4 → U(C8H8)2 + 4 KCl.[2]
Physical and chemical properties
Uranocene is paramagnetic, pyrophoric, and stable to hydrolysis. The η8-cyclooctatetraenyl groups are planar, as expected for a ring containing 10 π-electrons, and are mutually parallel, forming a sandwich containing the uranium atom. In the solid state, the rings are eclipsed, conferring D8h symmetry on the uranocene molecule. In solution the rings rotate with a low energy barrier.
Uranium-COT bond
The nature of the uranium-cyclooctatetraenyl bond is the subject of continuing research and debate [3]. UV-PES indicates the bonding in uranocene has contributions from 5f and 6d orbitals.
Analogous compounds
Some examples of analogous compounds of the form M(C8H8)2 exist for M = (Nd, Tb, Pu, Pa, Np, Th, and Yb). Extensions include the air-stable derivative U(C8H4Ph4)2 and the cycloheptatrienyl species [U(C7H7)2]−.[4]
References
- ^ A. Streitwieser and U. Mueller-Westerhoff (1968). "Bis(cyclooctatetraenyl)uranium (uranocene). A new class of sandwich complexes that utilize atomic f orbitals". J. Am. Chem. Soc. 90 (26): 7364–7364. doi:10.1021/ja01028a044.
- ^ J. S. Hager, J. Zahardis, R. M. Pagni, R. N. Compton and J. Li (2004). "Raman under nitrogen. The high-resolution Raman spectroscopy of crystalline uranocene, thorocene, and ferrocene". The Journal of Chemical Physics. 120 (6): 2708–2718. doi:10.1063/1.1637586.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lanthanides & Actinides: Organoactinides
- ^ D. Seyferth (2004). "Uranocene. The First Member of a New Class of Organometallic Derivatives of the f Elements". Organometallics. 23 (15): 3562–3583. doi:10.1021/om0400705.
Further reading
- The f elements, Nikolas Kaltsoyannis and Peter Scott. ISBN 0-19-850467-5
- Chemistry of the Elements, N. N. Greenwood and A. Earnshaw. ISBN 0-08-022057-6