Wet sulfuric acid process
This article needs additional citations for verification. (September 2011) |
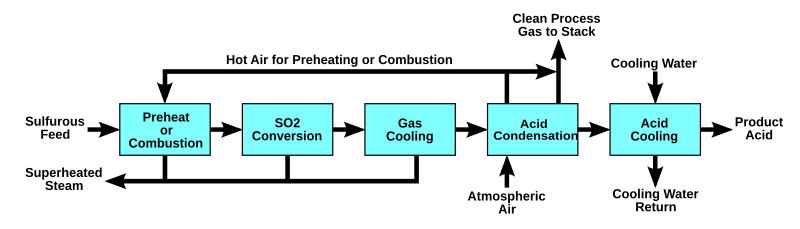
The wet sulfuric acid process (WSA process) is one of the key gas desulfurization processes on the market today. Since the Danish catalyst company Haldor Topsoe introduced and patented this technology in the late 1980s, it has been recognised as an efficient process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4), with simultaneous production of high pressure steam. The WSA process is applied in all industries where removal of sulfur is an issue.
The wet catalysis process is especially suited for processing one or more sulfur containing streams such as.:[1]
- H2S gas from e.g. amine gas treating unit
- Off-gas from sour water stripper (SWS gas)
- Off-gas from Rectisol
- Spent acid from an Alkylation unit
- Claus process tail gas
- Heavy residue or petcoke-fired utility boiler off-gas
- Boiler flue gases from various processes SNOX flue gas desulfurisation
- Metallurgical process gas
- Production of sulfuric acid
The process
- The main reactions in the WSA process
- Combustion: H2S + 1.5 O2 = H2O + SO2 + 518 kJ/mole
- Oxidation: SO2 + ½O2 = SO3 + 99 kJ/mole (in the presence of a vanadium (V) oxide catalyst)
- Hydration: SO3 + H2O = H2SO4 (g) + 101 kJ/mole
- Condensation: H2SO4 (g) = H2SO4 (l) + 90 kJ/mole
The energy released by the above-mentioned reactions is used for steam production. Approximately 2–3 ton high-pressure steam per ton of acid produced.
Industrial applications
Industries where WSA process plants are installed:
- Refinery and petrochemical industry
- Metallurgy industry
- Coal-based industry (coking and gasification)
- Power industry
- Viscose industry
- Sulfuric acid industry
WSA for gasifiers
The acid gas coming from a Rectisol-, Selexol-, amine gas treating or similar installed after the gasifier contains H2S, COS and hydrocarbons in addition to CO2. These gases were previously often flared and vented to the atmosphere, but now the acid gas requires purification in order not to affect the environment with SO2 emission. Not only can the WSA process meet the demands of SO2 removal, the process also accepts a wide range of feed-gas compositions.
The WSA plant provides a high sulfur recovery and the heat recovered causes a substantial steam production. The heat recovery rate is high and the cooling water consumption low, resulting in superior cost performance of this process.[citation needed]
Why use the WSA process in connection with gasification?
- flexibility in feed composition
- more than 99% of the sulfur is recovered as concentrated sulfuric acid of commercial grade
- attractive operating economy
- simple layout, simple operation[citation needed]
Examples of WSA process for gasification
Example 1:
- Feed-gas flow: 14,000 Nm3/h
- Composition [vol %]: 5.8% H2S, 1.2% COS, 9.7% HC and 77.4% CO2
- SOx concentration [vol %]: 1.58%
- H2SO4 production: 106 MTPD
- Steam production: 53 ton/h
- Cooling water consumption: 8 m3/ton acid (delta T = 10 °C)
- Fuel consumption: 1,000 Nm3/h (LHV = 2,821 kcal/Nm3)
Example 2: A sulfur plant in China will be built in connection with an ammonia plant, producing 500 kilotons/annum of ammonia for fertilizer production [2]
Spent acid regeneration and production of sulfuric acid
The WSA process can also be used for production of sulfuric acid from sulfur burning or for regeneration of the spent acid from e.g. alkylation plants. Wet catalysis processes differ from other contact sulfuric acid processes in that the feed gas contains excess moisture when it comes into contact with the catalyst. The sulfur trioxide formed by catalytic oxidation of the sulfur dioxide reacts instantly with the moisture to produce sulfuric acid in the vapour phase to an extent determined by the temperature. Liquid acid is subsequently formed by condensation of the sulfuric acid vapour and not by absorption of the sulfur trioxide in concentrated sulfuric acid, as is the case in contact processes based on dry gases.
The concentration of the product acid depends on the H2O/SO3 ratio in the catalytically converted gases and on the condensation temperature.[3][4]
The combustion gases are cooled to the converter inlet temperature of about 420–440 °C. To process these wet gases in a conventional cold-gas contact process (DCDA) plant would necessitate cooling and drying of the gas to remove all moisture. Therefore the WSA process is in many cases a more cost-efficient way of producing sulfuric acid.
About 80% to 85% of the world’s sulfur production is used to manufacture sulfuric acid. 50% of the world’s sulfuric acid production is used in fertilizer production, mainly to convert phosphates to water-soluble forms, according to the Fertilizer Manual, published jointly by the United Nations Industrial Development Organization (UNIDO) and the International Fertilizer Development Center. [5]
References
- ^ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics (2nd ed.). Marcel Dekker, Inc. ISBN 0-8247-7150-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ [1]; World Fuels
- ^ Sulphur recovery; (2007). The Process Principles in sulphur recovery by the WSA process.). Denmark: Jens Kristen Laursen, Haldor Topsoe A/S. Reprinted from Hydrocarbonengineering August 2007
- ^ U.H.F Sander, H. Fischer, U. Rothe, R. Kola (1984). Sulphur, Sulphur Dioxide and Sulphuric Acid (1st ed.). The British Sulphur Corporation Limited. ISBN 0-902777-64-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ [2]; (July 2008). IFDC FOCUS ON FERTILIZERS AND FOOD SECURITY,Issue 4; Global Shortage of Sulfuric Acid Contributes to Rising Fertilizer Costs Archived 2009-01-06 at the Wayback Machine