YOYO-1
| Names | |
|---|---|
| IUPAC name
{1,1'-(4,4,8,8-tetramethyl-4,8-diazaundecamethylene)bis[4-[(3-methylbenzo-1,3-oxazol-2-yl)methylidene]-l,4-dihydroquinolinium] tetraiodide}
| |
| Other names
YOYO, YOYO-1, YoYo-1
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C49H58I4N6O2 | |
| Molar mass | 1270.642 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
YOYO-1 is a green fluorescent dye used in DNA staining.[1] It belongs to the family of monomethine cyanine dyes and is a tetracationic homodimer of Oxazole Yellow (abbreviated YO, hence the name YOYO), typically available as tetraiodide salt. In aqueous buffer, free YOYO-1 dye (absorption: λmax 458 nm, emission: λmax 564 nm) has very low fluorescence quantum yield, however the intensity of fluorescence increases 3200 times upon binding through bis-intercalation to double-stranded DNA (absorption: λmax 489 nm, emission: λmax 509 nm).[2]
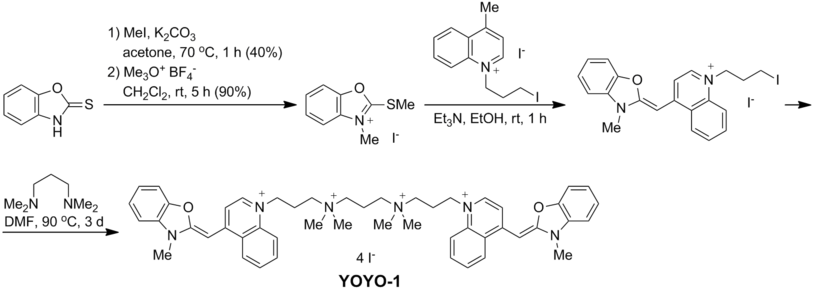
Synthesis
YOYO-1 is prepared by alkylation of N,N,N’,N’-tetramethyl-1,3-propanediamine with 2 equivalents of N-(3-iodopropyl) analogue of Oxazole Yellow,[2] which is available in three steps from 2-mercaptobenzoxazole:[3]
References
- ^ Bennink, ML; Schärer, OD; Kanaar, R; Sakata-Sogawa, K; et al. (June 1999). "Single-molecule manipulation of double-stranded DNA using optical tweezers: Interaction studies of DNA with RecA and YOYO-1". Cytometry Part A. 36 (3): 200–208. doi:10.1002/(SICI)1097-0320(19990701)36:3<200::AID-CYTO9>3.0.CO;2-T.
- ^ a b Rye, HS; Yue, S; Wemmer, DE; Quesada, MA; et al. (1992). "Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications". Nucleic Acids Research. 20 (11): 2803–2812. doi:10.1093/nar/20.11.2803.
- ^ WO 2010141833, Lee Josephson; Elisabeth Garanger & Scott Hilderbrand et al., "Vital fluorochrome conjugates and methods of use", published 2010-12-09, assigned to The General Hospital Corp
