β-Hydride elimination
This article needs additional citations for verification. (August 2012) |
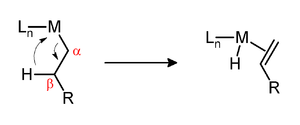
β-Hydride elimination is a reaction in which a metal-alkyl centre is converted into the corresponding metal-hydride-alkene.[1] β-Hydride elimination can also occur for many alkoxide complexes as well. The main requirements are that the alkyl group possess a C-H bond β to the metal and that the metal be coordinatively unsaturated. Thus, metal-butyl complexes are susceptible to this reaction whereas metal-methyl complexes are not. The complex must have an empty (or vacant) site cis to the alkyl group for this reaction to occur. β-Hydride elimination, which can be desirable or undesirable, affects the behavior of many organometallic complexes.
Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0 metals alkyls are generally more stable to β-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available.[2]
Role of β-hydride elimination
[edit]The Shell higher olefin process relies on β-hydride elimination to produce α-olefins which are used to produce detergents.
β-Hydride elimination interferes with the Ziegler–Natta polymerization, leading to decreased molecular weight.[3] The production of branched polymers from ethylene relies on chain walking, a key step of which is β-hydride elimination.
Nickel- and palladium-catalyzed couplings mainly focus on aryl-aryl couplings. Aryl-alkyl and especially alkyl-alkyl couplings are less successful because of β-hydride elimination can lower the yield.
In Hydroformylation, β-hydride elimination can act as a side reaction that influences product regioselectivity.[4] For example, in the hydroformylation of open chain unsaturated ethers, it reverses the formation of branched metal-alkyl intermediates at high temperatures, leading to a greater yield of linear products.[5]
β-Hydride elimination is one step in the synthesis of some metal hydrides. For instance in the synthesis of RuHCl(CO)(PPh3)3 from ruthenium trichloride, triphenylphosphine and 2-methoxyethanol, an intermediate alkoxide complex undergoes a β-hydride elimination to form the hydride ligand and the pi-bonded aldehyde which then is later converted into the carbonyl (carbon monoxide) ligand.
Mechanism
[edit]
β-Hydride elimination transforms a metal-alkyl complex into an metal-hydrido-alkene complex.[6] Starting with an unsaturated complex, the transformation proceeds in stages: 1) Dissociation of a ligand from a metal alkyl complex, yielding a coordinatively unsaturated derivative. 2) Alignment of the beta hydrogen. In this step, a vacant site on the metal forms an agostic complex by binding a C-H bond of the alkyl (or alkoxide).[7][8][9] 3) Hydride Transfer/Alkene Formation. In this step, the M-H bond forms concomitant with cleavage of a C-H bond and the development of a double bond in what was once an alkyl (or alkoxide) ligand.[9] The resulting metal hydride can eliminate the alkene ligand. The transition state for this β-hydride elimination involves a 4-membered ring.[10][11]
Non-dissociative
[edit]Especially for Pt(II) complexes, β-hydride eliminations may occur without the dissociation of an ancillary ligand.[12][13][14][15] This was suggested primarily based on the observed order of the L-type ligand in the rate law derived from kinetic studies. This mechanism appears to be operative for the minority of reactions studied.
Structure-Reactivity Relationships
[edit]Relative to an arbitrary reference complex, β-hydride elimination is faster in a complex with the following characteristics:
- More electron-deficient metal center. This can be as a result of less donating ancillary ligands.[16][17]
- More labile ancillary ligands, such as weakly coordinating (e.g. solvent) or sterically demanding ligands. [18][9][17]
- The abstracted H is more hydridic (has a higher pKa).[19][20]
Avoiding β-hydride elimination
[edit]Several strategies exist for avoiding β-hydride elimination. The most common strategy is to employ alkyl ligands that lack hydrogen atoms at the β position. Common substituents include methyl and neopentyl. β-Hydride elimination is also inhibited when the reaction would produce a strained alkene. This situation is illustrated by the stability of metal complexes containing norbornyl ligands, where the β-hydride elimination product would violate Bredt's rule.[21]
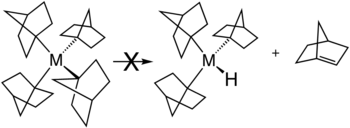
Further reading
[edit]Dissociation-induced β-hydride eliminations.[9][22][23][24]
β-Hydride elimination involving metal alkoxide and amido complexes.[25][26][27][28][29][30]
References
[edit]- ^ Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ^ Crabtree, Robert H. (2005). The organometallic chemistry of the transition metals (4th ed.). Hoboken, N.J.: John Wiley. p. 58. ISBN 0-471-66256-9. OCLC 61520528.
- ^ Burger, Barbara J.; Thompson, Mark E.; Cotter, W. Donald; Bercaw, John E. (1990-02-01). "Ethylene insertion and .beta.-hydrogen elimination for permethylscandocene alkyl complexes. A study of the chain propagation and termination steps in Ziegler-Natta polymerization of ethylene". Journal of the American Chemical Society. 112 (4): 1566–1577. Bibcode:1990JAChS.112.1566B. doi:10.1021/ja00160a041. ISSN 0002-7863.
- ^ Zhang, Baoxin; Peña Fuentes, Dilver; Börner, Armin (2022-12-02). "Hydroformylation". ChemTexts. 8 (1): 2. Bibcode:2022ChTxt...8....2Z. doi:10.1007/s40828-021-00154-x. ISSN 2199-3793.
- ^ Lazzaroni, Raffaello; Settambolo, Roberta; Uccello-Barretta, Gloria (1995-10-01). ".beta.-Hydride Elimination and Regioselectivity in the Rhodium-Catalyzed Hydroformylation of Open Chain Unsaturated Ethers". Organometallics. 14 (10): 4644–4650. doi:10.1021/om00010a031. ISSN 0276-7333.
- ^ Hartwig, John F. (2010). Organotransition metal chemistry: from bonding to Catalysis. Sausalito, Calif: University Science Books. ISBN 978-1-891389-53-5. OCLC 310401036.
- ^ Lu, Xiyan (2005-06-01). "Control of the β-Hydride Elimination Making Palladium-Catalyzed Coupling Reactions more Diversified". Topics in Catalysis. 35 (1): 73–86. doi:10.1007/s11244-005-3814-4. ISSN 1572-9028.
- ^ Spessard, Gary O.; Miessler, Gary L. (2016). Organometallic chemistry (3rd ed.). New York: Oxford University Press. ISBN 978-0-19-934267-9.
- ^ a b c d Theofanis, Patrick L.; Goddard, William A. III (2011-09-26). "Understanding β-Hydride Eliminations from Heteroatom Functional Groups". Organometallics. 30 (18): 4941–4948. doi:10.1021/om200542w. ISSN 0276-7333. Cite error: The named reference ":0" was defined multiple times with different content (see the help page).
- ^ Pudasaini, Bimal; Janesko, Benjamin G. (2012-06-25). "Computational Mechanistic Study of Stereoselective Suzuki Coupling of an α-Cyano-Activated Secondary Alkyl". Organometallics. 31 (12): 4610–4618. doi:10.1021/om300455g. ISSN 0276-7333.
- ^ Theofanis, Patrick L.; Goddard, William A. (2011-09-26). "Understanding β-Hydride Eliminations from Heteroatom Functional Groups". Organometallics. 30 (18): 4941–4948. doi:10.1021/om200542w. ISSN 0276-7333.
- ^ Nuzzo, Ralph G.; McCarthy, Thomas J.; Whitesides, George M. (June 1981). "Thermal decomposition of di(cycloalkyl)bis(triethylphosphine)platinum(II) complexes". Journal of the American Chemical Society. 103 (12): 3404–3410. Bibcode:1981JAChS.103.3404N. doi:10.1021/ja00402a026. ISSN 0002-7863.
- ^ McCarthy, Thomas J.; Nuzzo, Ralph G.; Whitesides, George M. (June 1981). "Mechanisms of thermal decomposition of diethylbis(triethylphosphine)platinum(II)". Journal of the American Chemical Society. 103 (12): 3396–3403. Bibcode:1981JAChS.103.3396M. doi:10.1021/ja00402a025. ISSN 0002-7863.
- ^ McDermott, Joseph X.; White, John F.; Whitesides, George M. (October 1976). "Thermal decomposition of bis(phosphine)platinum(II) metallocycles". Journal of the American Chemical Society. 98 (21): 6521–6528. Bibcode:1976JAChS..98.6521M. doi:10.1021/ja00437a018. ISSN 0002-7863.
- ^ Whitesides, George M.; Gaasch, John F.; Stedronsky, Erwin R. (July 1972). "Mechanism of thermal decomposition of dibutylbis(triphenylphosphine)platinum(II)". Journal of the American Chemical Society. 94 (15): 5258–5270. Bibcode:1972JAChS..94.5258W. doi:10.1021/ja00770a021. ISSN 0002-7863.
- ^ Alibrandi, Giuseppe; Monsu Scolaro, Luigi; Minniti, Domenico; Romeo, Raffaello (September 1990). "Kinetic study of .beta.-hydride elimination of monoalkyl complexes of platinum(II): effects of varying the alkyl chain length or the cis group in the reaction of cis-bis(triethylphosphine)(alkyl)(halo or pseudohalo)platinum(II) complexes". Inorganic Chemistry. 29 (18): 3467–3472. doi:10.1021/ic00343a037. ISSN 0020-1669.
- ^ a b Bogdos, Michael K.; Stepanović, Olivera; Bismuto, Alessandro; Luraschi, Mauro G.; Morandi, Bill (2022-09-12). "Mechanistically informed selection rules for competing β-hydride and β-heteroatom eliminations". Nature Synthesis. 1 (10): 787–793. Bibcode:2022NatSy...1..787B. doi:10.1038/s44160-022-00145-x. ISSN 2731-0582.
- ^ Romeo, Raffaello; Alibrandi, Giuseppe; Scolaro, Luigi Monsu (October 1993). "Kinetic study of .beta.-hydride elimination from monoalkyl solvento complexes of platinum(II)". Inorganic Chemistry. 32 (22): 4688–4694. doi:10.1021/ic00074a008. ISSN 0020-1669.
- ^ Chirik, Paul J.; Bercaw, John E. (2005-10-01). "Cyclopentadienyl and Olefin Substituent Effects on Insertion and β-Hydrogen Elimination with Group 4 Metallocenes. Kinetics, Mechanism, and Thermodynamics for Zirconocene and Hafnocene Alkyl Hydride Derivatives". Organometallics. 24 (22): 5407–5423. doi:10.1021/om0580351. ISSN 0276-7333.
- ^ Burger, Barbara J.; Thompson, Mark E.; Cotter, W. Donald; Bercaw, John E. (February 1990). "Ethylene insertion and .beta.-hydrogen elimination for permethylscandocene alkyl complexes. A study of the chain propagation and termination steps in Ziegler-Natta polymerization of ethylene". Journal of the American Chemical Society. 112 (4): 1566–1577. Bibcode:1990JAChS.112.1566B. doi:10.1021/ja00160a041. ISSN 0002-7863.
- ^ Bower, Barton K.; Tennent, Howard G. (1972). "Transition metal bicyclo[2.2.1]hept-1-yls". J. Am. Chem. Soc. 94 (7): 2512–2514. Bibcode:1972JAChS..94.2512B. doi:10.1021/ja00762a056.
- ^ Lloyd-Jones, Guy C.; Slatford, Paul A. (2004-03-01). "Unusually Large Primary 2 H/H Kinetic Isotope Effects Accompanying a syn -β-H Elimination Reaction in a σ-Alkyl−Palladium Complex". Journal of the American Chemical Society. 126 (9): 2690–2691. Bibcode:2004JAChS.126.2690L. doi:10.1021/ja039349n. ISSN 0002-7863. PMID 14995172.
- ^ Keinan, Ehud; Kumar, Sandeep; Dangur, Vered; Vaya, Jacob (November 1994). "Evidence for a Cyclic Mechanism in (.eta.3-Allyl)palladium Chemistry. Promotion of .beta.-Hydride Elimination by Unsaturated Organometallics". Journal of the American Chemical Society. 116 (24): 11151–11152. Bibcode:1994JAChS.11611151K. doi:10.1021/ja00103a038. ISSN 0002-7863.
- ^ Alexanian, Erik J.; Hartwig, John F. (2008-11-19). "Mechanistic Study of β-Hydrogen Elimination from Organoplatinum(II) Enolate Complexes". Journal of the American Chemical Society. 130 (46): 15627–15635. Bibcode:2008JAChS.13015627A. doi:10.1021/ja8056908. ISSN 0002-7863. PMC 2819484. PMID 18954048.
- ^ Cornella, Josep; Gómez-Bengoa, Enrique; Martin, Ruben (2013-02-06). "Combined Experimental and Theoretical Study on the Reductive Cleavage of Inert C–O Bonds with Silanes: Ruling out a Classical Ni(0)/Ni(II) Catalytic Couple and Evidence for Ni(I) Intermediates". Journal of the American Chemical Society. 135 (5): 1997–2009. Bibcode:2013JAChS.135.1997C. doi:10.1021/ja311940s. ISSN 0002-7863.
- ^ Mueller, Jaime A.; Goller, Christopher P.; Sigman, Matthew S. (2004-08-01). "Elucidating the Significance of β-Hydride Elimination and the Dynamic Role of Acid/Base Chemistry in a Palladium-Catalyzed Aerobic Oxidation of Alcohols". Journal of the American Chemical Society. 126 (31): 9724–9734. Bibcode:2004JAChS.126.9724M. doi:10.1021/ja047794s. ISSN 0002-7863. PMC 2720309. PMID 15291576.
- ^ Rauch, Michael; Luo, Jie; Avram, Liat; Ben-David, Yehoshoa; Milstein, David (2021-03-05). "Mechanistic Investigations of Ruthenium Catalyzed Dehydrogenative Thioester Synthesis and Thioester Hydrogenation". ACS Catalysis. 11 (5): 2795–2807. doi:10.1021/acscatal.1c00418. ISSN 2155-5435. PMC 7976608. PMID 33763290.
- ^ Barrera, Joseph; Orth, Stephen D.; Harman, W. Dean (August 1992). ".beta.-Hydride elimination for an amine ligand and the microscopic reverse: the first report of a cis-iminium hydride in equilibrium with its amine precursor". Journal of the American Chemical Society. 114 (18): 7316–7318. Bibcode:1992JAChS.114.7316B. doi:10.1021/ja00044a065. ISSN 0002-7863.
- ^ Zhao, Jing; Hesslink, Heather; Hartwig, John F. (2001-08-01). "Mechanism of β -Hydrogen Elimination from Square Planar Iridium(I) Alkoxide Complexes with Labile Dative Ligands". Journal of the American Chemical Society. 123 (30): 7220–7227. Bibcode:2001JAChS.123.7220Z. doi:10.1021/ja010417k. ISSN 0002-7863. PMID 11472149.
- ^ Hartwig, John F. (1996-01-01). "Directly-Observed β-Hydrogen Elimination of a Late Transition Metal Amido Complex and Unusual Fate of Imine Byproducts". Journal of the American Chemical Society. 118 (29): 7010–7011. Bibcode:1996JAChS.118.7010H. doi:10.1021/ja961439n. ISSN 0002-7863.
