Paeoniflorin
Appearance
(Redirected from Peoniflorin)
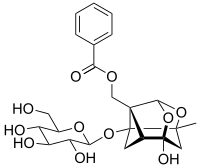
| |
| Names | |
|---|---|
| Other names
Paeonia moutan
Paeony root Peoniflorin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.041.327 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H28O11 | |
| Molar mass | 480.466 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Paeoniflorin is a chemical compound which is one of the major constituents of an herbal medicine derived from Paeonia lactiflora.[1] It can also be isolated from the fresh water fern Salvinia molesta.[2]
In Paeonia, it can form new compounds with addition of phenolic substituents.[3] In a study in female rats, paeoniflorin was found to inhibit the production of testosterone within the ovaries, however does not significantly affect the production of Estradiol.[4] In mice, paeoniflorin was shown to protect against neuroinflammation and depression-like behavior induced by IFN alpha.[5]
References
[edit]- ^ Yan, D.; Saito, K.; Ohmi, Y.; Fujie, N.; Ohtsuka, K. (2004). "Paeoniflorin, a novel heat shock protein–inducing compound". Cell Stress & Chaperones. 9 (4): 378–89. doi:10.1379/CSC-51R.1. PMC 1065277. PMID 15633296.
- ^ Choudhary, M. I.; Naheed, N.; Abbaskhan, A.; Musharraf, S. G.; Siddiqui, H.; Atta-Ur-Rahman (2008). "Phenolic and other constituents of fresh water fern Salvinia molesta". Phytochemistry. 69 (4): 1018–1023. Bibcode:2008PChem..69.1018C. doi:10.1016/j.phytochem.2007.10.028. PMID 18177906.
- ^ Tanaka, T.; Kataoka, M.; Tsuboi, N.; Kouno, I. (2000). "New monoterpene glycoside esters and phenolic constituents of Paeoniae radix, and increase of water solubility of proanthocyanidins in the presence of paeoniflorin". Chemical & Pharmaceutical Bulletin. 48 (2): 201–207. doi:10.1248/cpb.48.201. PMID 10705504.
- ^ Takeuchi, Toru; Nishii, Osamu; Okamura, Takashi; Yaginuma, Tsutomu (1991). "Effect of Paeoniflorin, Glycyrrhizin and Glycyrrhetic acid on Ovarian Androgen Production". The American Journal of Chinese Medicine. 19 (1): 73–8. doi:10.1142/S0192415X91000119. PMID 1897494.
- ^ Paeoniflorin ameliorates interferon-alpha-induced neuroinflammation and depressive-like behaviors in mice.
