Coronavirus nucleocapsid protein: Difference between revisions
Citation bot (talk | contribs) Add: isbn, pmc, pages, doi-access, pmid, authors 1-1. Removed proxy/dead URL that duplicated identifier. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Headbomb | Linked from Wikipedia:WikiProject_Academic_Journals/Journals_cited_by_Wikipedia/Sandbox | #UCB_webform_linked 886/1012 |
I added some information in the Evolution and Conservation section that the N-protein is well conserved, it does not recombine frequently and that it is a good target for vaccines |
||
| Line 57: | Line 57: | ||
==Evolution and conservation== |
==Evolution and conservation== |
||
The structures of N proteins from different coronaviruses, particularly the C-terminal domains, appear to be well conserved.<ref name=chang_2014 /><ref name=ye_2020 /> Similarities between the structure and topology of the N proteins of coronaviruses and [[arterivirus]]es suggest a common evolutionary origin and supports the classification of these two groups in the common order ''[[Nidovirales]]''.<ref name=chang_2014 /><ref name=mcbride_2014 /> |
The sequences and structures of N proteins from different coronaviruses, particularly the C-terminal domains, appear to be well conserved.<ref name=chang_2014 /><ref name=ye_2020 /><ref name=":0">{{Cite journal|last=Nikolaidis|first=Marios|last2=Markoulatos|first2=Panayotis|last3=Van de Peer|first3=Yves|last4=Oliver|first4=Stephen G|last5=Amoutzias|first5=Grigorios D|date=2021-10-12|editor-last=Hepp|editor-first=Crystal|title=The neighborhood of the Spike gene is a hotspot for modular intertypic homologous and non-homologous recombination in Coronavirus genomes|url=https://academic.oup.com/mbe/advance-article/doi/10.1093/molbev/msab292/6382323|journal=Molecular Biology and Evolution|language=en|pages=msab292|doi=10.1093/molbev/msab292|issn=0737-4038}}</ref> Similarities between the structure and topology of the N proteins of coronaviruses and [[arterivirus]]es suggest a common evolutionary origin and supports the classification of these two groups in the common order ''[[Nidovirales]]''.<ref name=chang_2014 /><ref name=mcbride_2014 /> |
||
Examination of SARS-CoV-2 sequences collected during the [[Covid-19 pandemic]] found that [[missense mutation]]s were most common in the central linker region of the protein, suggesting this relatively unstructured region is more tolerant of mutations than the structured domains.<ref name=ye_2020 /> A separate study of SARS-CoV-2 sequences identified at least one site in the N protein under [[positive selection]].<ref name="cagliani_2020">{{cite journal |last1=Cagliani |first1=Rachele |last2=Forni |first2=Diego |last3=Clerici |first3=Mario |last4=Sironi |first4=Manuela |title=Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 |journal=Journal of Virology |date=June 2020 |volume=94 |issue=12 |pages=e00411-20 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }}</ref> |
Examination of SARS-CoV-2 sequences collected during the [[Covid-19 pandemic]] found that [[missense mutation]]s were most common in the central linker region of the protein, suggesting this relatively unstructured region is more tolerant of mutations than the structured domains.<ref name=ye_2020 /> A separate study of SARS-CoV-2 sequences identified at least one site in the N protein under [[positive selection]].<ref name="cagliani_2020">{{cite journal |last1=Cagliani |first1=Rachele |last2=Forni |first2=Diego |last3=Clerici |first3=Mario |last4=Sironi |first4=Manuela |title=Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 |journal=Journal of Virology |date=June 2020 |volume=94 |issue=12 |pages=e00411-20 |doi=10.1128/JVI.00411-20|pmid=32238584 |pmc=7307108 }}</ref> |
||
Since the N protein can generate a robust T-cell response and is also well conserved and does not appear to recombine frequently, it may be a good target for inclusion in the next generation of coronavirus vaccines. <ref>{{Cite journal|last=Grifoni|first=Alba|last2=Weiskopf|first2=Daniela|last3=Ramirez|first3=Sydney I.|last4=Mateus|first4=Jose|last5=Dan|first5=Jennifer M.|last6=Moderbacher|first6=Carolyn Rydyznski|last7=Rawlings|first7=Stephen A.|last8=Sutherland|first8=Aaron|last9=Premkumar|first9=Lakshmanane|last10=Jadi|first10=Ramesh S.|last11=Marrama|first11=Daniel|date=2020-06|title=Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals|url=https://linkinghub.elsevier.com/retrieve/pii/S0092867420306103|journal=Cell|language=en|volume=181|issue=7|pages=1489–1501.e15|doi=10.1016/j.cell.2020.05.015|pmc=PMC7237901|pmid=32473127}}</ref><ref>{{Cite journal|last=Dutta|first=Noton K.|last2=Mazumdar|first2=Kaushiki|last3=Gordy|first3=James T.|date=2020-06-16|editor-last=Dutch|editor-first=Rebecca Ellis|title=The Nucleocapsid Protein of SARS–CoV-2: a Target for Vaccine Development|url=https://journals.asm.org/doi/10.1128/JVI.00647-20|journal=Journal of Virology|language=en|volume=94|issue=13|doi=10.1128/JVI.00647-20|issn=0022-538X|pmc=PMC7307180|pmid=32546606}}</ref><ref name=":0" /> |
|||
==References== |
==References== |
||
Revision as of 17:28, 23 October 2021
| Nucleocapsid protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
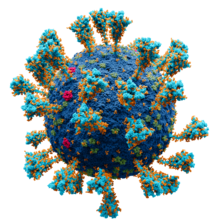 Model of the external structure of the SARS-CoV-2 virion.[1] The N protein, contained entirely within the virion, is not visible.
● Blue: envelope ● Turquoise: spike glycoprotein (S) ● Red: envelope proteins (E) ● Green: membrane proteins (M) ● Orange: glycans | |||||||||
| Identifiers | |||||||||
| Symbol | CoV_nucleocap | ||||||||
| Pfam | PF00937 | ||||||||
| InterPro | IPR001218 | ||||||||
| |||||||||
The nucleocapsid (N) protein is a protein that packages the positive-sense RNA genome of coronaviruses to form ribonucleoprotein structures enclosed within the viral capsid.[2][3] The N protein is the most highly expressed of the four major coronavirus structural proteins.[2] In addition to its interactions with RNA, N forms protein-protein interactions with the coronavirus membrane protein (M) during the process of viral assembly.[2][3] N also has additional functions in manipulating the cell cycle of the host cell.[3][4] The N protein is highly immunogenic and antibodies to N are found in patients recovered from SARS and Covid-19.[5]
Structure
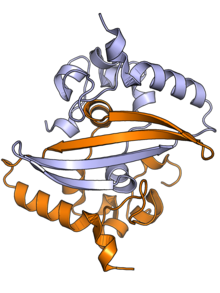
The N protein is composed of two main protein domains connected by an intrinsically disordered region (IDR) known as the linker region, with additional disordered segments at each terminus.[2][3] A third small domain at the C-terminal tail appears to have an ordered alpha helical secondary structure and may be involved in the formation of higher-order oligomeric assemblies.[6] In SARS-CoV, the causative agent of SARS, the N protein is 422 amino acid residues long[2] and in SARS-CoV-2, the causative agent of Covid-19, it is 419 residues long.[6][7]
Both the N-terminal and C-terminal domains are capable of binding RNA. The C-terminal domain forms a dimer that is likely to be the native functional state.[2] Parts of the IDR, particularly a conserved sequence motif rich in serine and arginine residues (the SR-rich region), may also be implicated in dimer formation, though reports on this vary.[2][3] Although higher-order oligomers formed through the C-terminal domain have been observed crystallographically, it is unclear if these structures have a physiological role.[2][8]
The C-terminal dimer has been structurally characterized by X-ray crystallography for several coronaviruses and has a highly conserved structure.[6] The N-terminal domain - sometimes known as the RNA-binding domain, though other parts of the protein also interact with RNA - has also been crystallized and has been studied by nuclear magnetic resonance spectroscopy in the presence of RNA.[9]
Post-translational modifications
The N protein is post-translationally modified by phosphorylation at sites located in the IDR, particularly in the SR-rich region.[2][10] In several coronaviruses, ADP-ribosylation of the N protein has also been reported.[11][10] With unclear functional significance, the SARS-CoV N protein has been observed to be SUMOylated and the N proteins of several coronaviruses including SARS-CoV-2 have been observed to be proteolytically cleaved.[10][12][13]
Expression and localization
The N protein is the most highly expressed in host cells of the four major structural proteins.[2] Like the other structural proteins, the gene encoding the N protein is located toward the 3' end of the genome.[3]
N protein is localized primarily to the cytoplasm.[3] In many coronaviruses, a population of N protein is localized to the nucleolus,[3][4][14] thought to be associated with its effects on the cell cycle.[4]
Function
Genome packaging and viral assembly

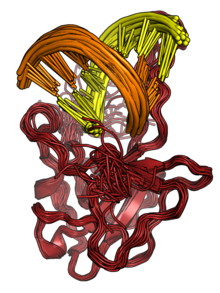
The N protein binds to RNA to form ribonucleoprotein (RNP) structures for packaging the genome into the viral capsid.[2][3] The RNP particles formed are roughly spherical and are organized in flexible helical structures inside the virus.[2][3] Formation of RNPs is thought to involve allosteric interactions between RNA and multiple RNA-binding regions of the protein.[2][8] Dimerization of N is important for assembly of RNPs. Encapsidation of the genome occurs through interactions between N and M.[2][3] N is essential for viral assembly.[3] N also serves as a chaperone protein for the formation of RNA structure in the genomic RNA.[3][8]
Genomic and subgenomic RNA synthesis
Synthesis of genomic RNA appears to involve participation by the N protein. N is physically colocalized with the viral RNA-dependent RNA polymerase early in the replication cycle and forms interactions with non-structural protein 3, a component of the replicase-transcriptase complex.[3] Although N appears to facilitate efficient replication of genomic RNA, it is not required for RNA transcription in all coronaviruses.[3][15] In at least one coronavirus, transmissible gastroenteritis virus (TGEV), N is involved in template switching in the production of subgenomic mRNAs, a process that is a distinctive feature of viruses in the order Nidovirales.[3][15][16]
Cell cycle effects
Coronaviruses manipulate the cell cycle of the host cell through various mechanisms. In several coronaviruses, including SARS-CoV, the N protein has been reported to cause cell cycle arrest in S phase through interactions with cyclin-CDK.[3][4] In SARS-CoV, a cyclin box-binding region in the N protein can serve as a cyclin-CDK phosphorylation substrate.[3] Trafficking of N to the nucleolus may also play a role in cell cycle effects.[4] More broadly, N may be involved in reduction of host cell protein translation activity.[3]
Immune system effects
The N protein is involved in viral pathogenesis via its effects on components of the immune system. In SARS-CoV,[3][17][18] MERS-CoV,[19] and SARS-CoV-2,[20] N has been reported as suppressing interferon responses.
Evolution and conservation
The sequences and structures of N proteins from different coronaviruses, particularly the C-terminal domains, appear to be well conserved.[2][6][21] Similarities between the structure and topology of the N proteins of coronaviruses and arteriviruses suggest a common evolutionary origin and supports the classification of these two groups in the common order Nidovirales.[2][3]
Examination of SARS-CoV-2 sequences collected during the Covid-19 pandemic found that missense mutations were most common in the central linker region of the protein, suggesting this relatively unstructured region is more tolerant of mutations than the structured domains.[6] A separate study of SARS-CoV-2 sequences identified at least one site in the N protein under positive selection.[22]
Since the N protein can generate a robust T-cell response and is also well conserved and does not appear to recombine frequently, it may be a good target for inclusion in the next generation of coronavirus vaccines. [23][24][21]
References
- ^ Solodovnikov, Alexey; Arkhipova, Valeria (2021-07-29). "Достоверно красиво: как мы сделали 3D-модель SARS-CoV-2" [Truly beautiful: how we made the SARS-CoV-2 3D model] (in Russian). N+1. Archived from the original on 2021-07-30. Retrieved 30 July 2021.
- ^ a b c d e f g h i j k l m n o p Chang, Chung-ke; Hou, Ming-Hon; Chang, Chi-Fon; Hsiao, Chwan-Deng; Huang, Tai-huang (March 2014). "The SARS coronavirus nucleocapsid protein – Forms and functions". Antiviral Research. 103: 39–50. doi:10.1016/j.antiviral.2013.12.009. PMC 7113676. PMID 24418573.
- ^ a b c d e f g h i j k l m n o p q r s t u McBride, Ruth; van Zyl, Marjorie; Fielding, Burtram (7 August 2014). "The Coronavirus Nucleocapsid Is a Multifunctional Protein". Viruses. 6 (8): 2991–3018. doi:10.3390/v6082991. PMC 4147684. PMID 25105276.
- ^ a b c d e Su, Mingjun; Chen, Yaping; Qi, Shanshan; Shi, Da; Feng, Li; Sun, Dongbo (5 November 2020). "A Mini-Review on Cell Cycle Regulation of Coronavirus Infection". Frontiers in Veterinary Science. 7: 586826. doi:10.3389/fvets.2020.586826. PMC 7674852. PMID 33251267.
- ^ Li, Dandan; Li, Jinming (20 April 2021). "Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective". Journal of Clinical Microbiology. 59 (5): e02160-20. doi:10.1128/JCM.02160-20. PMC 8091849. PMID 33318065.
- ^ a b c d e f Ye, Qiaozhen; West, Alan M. V.; Silletti, Steve; Corbett, Kevin D. (September 2020). "Architecture and self‐assembly of the SARS‐CoV ‐2 nucleocapsid protein". Protein Science. 29 (9): 1890–1901. doi:10.1002/pro.3909. PMC 7405475. PMID 32654247.
- ^ Shah, Vibhuti Kumar; Firmal, Priyanka; Alam, Aftab; Ganguly, Dipyaman; Chattopadhyay, Samit (7 August 2020). "Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past". Frontiers in Immunology. 11: 1949. doi:10.3389/fimmu.2020.01949. PMC 7426442. PMID 32849654.
- ^ a b c Chang, Chung-ke; Lo, Shou-Chen; Wang, Yong-Sheng; Hou, Ming-Hon (April 2016). "Recent insights into the development of therapeutics against coronavirus diseases by targeting N protein". Drug Discovery Today. 21 (4): 562–572. doi:10.1016/j.drudis.2015.11.015. PMC 7108309. PMID 26691874.
- ^ a b Dinesh, Dhurvas Chandrasekaran; Chalupska, Dominika; Silhan, Jan; Koutna, Eliska; Nencka, Radim; Veverka, Vaclav; Boura, Evzen (2 December 2020). "Structural basis of RNA recognition by the SARS-CoV-2 nucleocapsid phosphoprotein". PLOS Pathogens. 16 (12): e1009100. doi:10.1371/journal.ppat.1009100. PMC 7735635. PMID 33264373.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Fung, To Sing; Liu, Ding Xiang (June 2018). "Post-translational modifications of coronavirus proteins: roles and function". Future Virology. 13 (6): 405–430. doi:10.2217/fvl-2018-0008. PMC 7080180. PMID 32201497.
- ^ Grunewald, Matthew E.; Fehr, Anthony R.; Athmer, Jeremiah; Perlman, Stanley (April 2018). "The coronavirus nucleocapsid protein is ADP-ribosylated". Virology. 517: 62–68. doi:10.1016/j.virol.2017.11.020. PMC 5871557. PMID 29199039.
- ^ Lutomski, Corinne A.; El-Baba, Tarick J.; Bolla, Jani R.; Robinson, Carol V. (2021-08-23). "Multiple Roles of SARS-CoV-2 N Protein Facilitated by Proteoform-Specific Interactions with RNA, Host Proteins, and Convalescent Antibodies". JACS Au. 1 (8): 1147–1157. doi:10.1021/jacsau.1c00139. ISSN 2691-3704. PMC 8231660. PMID 34462738.
- ^ Meyer, Bjoern; Chiaravalli, Jeanne; Gellenoncourt, Stacy; Brownridge, Philip; Bryne, Dominic P.; Daly, Leonard A.; Grauslys, Arturas; Walter, Marius; Agou, Fabrice; Chakrabarti, Lisa A.; Craik, Charles S. (December 2021). "Characterising proteolysis during SARS-CoV-2 infection identifies viral cleavage sites and cellular targets with therapeutic potential". Nature Communications. 12 (1): 5553. doi:10.1038/s41467-021-25796-w. ISSN 2041-1723. PMID 34548480.
- ^ Masters, Paul S. (2006). "The Molecular Biology of Coronaviruses". Advances in Virus Research. 66: 193–292. doi:10.1016/S0065-3527(06)66005-3. ISBN 9780120398690. PMC 7112330. PMID 16877062.
- ^ a b Zúñiga, Sonia; Cruz, Jazmina L. G.; Sola, Isabel; Mateos-Gómez, Pedro A.; Palacio, Lorena; Enjuanes, Luis (15 February 2010). "Coronavirus Nucleocapsid Protein Facilitates Template Switching and Is Required for Efficient Transcription". Journal of Virology. 84 (4): 2169–2175. doi:10.1128/JVI.02011-09. PMC 2812394. PMID 19955314.
- ^ Sola, Isabel; Almazán, Fernando; Zúñiga, Sonia; Enjuanes, Luis (9 November 2015). "Continuous and Discontinuous RNA Synthesis in Coronaviruses". Annual Review of Virology. 2 (1): 265–288. doi:10.1146/annurev-virology-100114-055218. PMC 6025776. PMID 26958916.
- ^ Spiegel, Martin; Pichlmair, Andreas; Martínez-Sobrido, Luis; Cros, Jerome; García-Sastre, Adolfo; Haller, Otto; Weber, Friedemann (15 February 2005). "Inhibition of Beta Interferon Induction by Severe Acute Respiratory Syndrome Coronavirus Suggests a Two-Step Model for Activation of Interferon Regulatory Factor 3". Journal of Virology. 79 (4): 2079–2086. doi:10.1128/JVI.79.4.2079-2086.2005. PMC 546554. PMID 15681410.
- ^ Kopecky-Bromberg, Sarah A.; Martínez-Sobrido, Luis; Frieman, Matthew; Baric, Ralph A.; Palese, Peter (15 January 2007). "Severe Acute Respiratory Syndrome Coronavirus Open Reading Frame (ORF) 3b, ORF 6, and Nucleocapsid Proteins Function as Interferon Antagonists". Journal of Virology. 81 (2): 548–557. doi:10.1128/JVI.01782-06. PMC 1797484. PMID 17108024.
- ^ Chang, Chi-You; Liu, Helene Minyi; Chang, Ming-Fu; Chang, Shin C. (16 June 2020). "Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Suppresses Type I and Type III Interferon Induction by Targeting RIG-I Signaling". Journal of Virology. 94 (13): e00099-20. doi:10.1128/JVI.00099-20. PMC 7307178. PMID 32295922.
- ^ Mu, Jingfang; Fang, Yaohui; Yang, Qi; Shu, Ting; Wang, An; Huang, Muhan; Jin, Liang; Deng, Fei; Qiu, Yang; Zhou, Xi (December 2020). "SARS-CoV-2 N protein antagonizes type I interferon signaling by suppressing phosphorylation and nuclear translocation of STAT1 and STAT2". Cell Discovery. 6 (1): 65. doi:10.1038/s41421-020-00208-3. PMC 7490572. PMID 32953130.
- ^ a b Nikolaidis, Marios; Markoulatos, Panayotis; Van de Peer, Yves; Oliver, Stephen G; Amoutzias, Grigorios D (2021-10-12). Hepp, Crystal (ed.). "The neighborhood of the Spike gene is a hotspot for modular intertypic homologous and non-homologous recombination in Coronavirus genomes". Molecular Biology and Evolution: msab292. doi:10.1093/molbev/msab292. ISSN 0737-4038.
- ^ Cagliani, Rachele; Forni, Diego; Clerici, Mario; Sironi, Manuela (June 2020). "Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2". Journal of Virology. 94 (12): e00411-20. doi:10.1128/JVI.00411-20. PMC 7307108. PMID 32238584.
- ^ Grifoni, Alba; Weiskopf, Daniela; Ramirez, Sydney I.; Mateus, Jose; Dan, Jennifer M.; Moderbacher, Carolyn Rydyznski; Rawlings, Stephen A.; Sutherland, Aaron; Premkumar, Lakshmanane; Jadi, Ramesh S.; Marrama, Daniel (2020-06). "Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals". Cell. 181 (7): 1489–1501.e15. doi:10.1016/j.cell.2020.05.015. PMC 7237901. PMID 32473127.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Dutta, Noton K.; Mazumdar, Kaushiki; Gordy, James T. (2020-06-16). Dutch, Rebecca Ellis (ed.). "The Nucleocapsid Protein of SARS–CoV-2: a Target for Vaccine Development". Journal of Virology. 94 (13). doi:10.1128/JVI.00647-20. ISSN 0022-538X. PMC 7307180. PMID 32546606.
{{cite journal}}: CS1 maint: PMC format (link)

