Nitrogen-13: Difference between revisions
Citation bot (talk | contribs) Alter: template type. Add: doi, journal. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Isotopes of nitrogen | #UCB_Category 12/20 |
Prime Lemur (talk | contribs) →Production: Added proper section-lede. Added "N-13 as medical imaging tracer" narrative. Added references I relied on. Moved existing text into new narrative order. This is well outside of my area of interest, so someone with sharp eyes needed to correct any mistakes I've made, as well as reword & better integrate pre-existing text. Tags: Mobile edit Mobile web edit Advanced mobile edit |
||
| Line 40: | Line 40: | ||
==Production== |
==Production== |
||
Nitrogen-13 is used in medical PET imaging in the form of <sup>13</sup>N-labelled ammonia. It can be produced with a medical cyclotron, using a target of pure water with a trace amount of ethanol. The reactants are oxygen-16 and a proton, and the products are nitrogen-13 and an alpha particle (helium-4). |
|||
:<sup>1</sup>H + <sup>16</sup>O → <sup>13</sup>N + <sup>4</sup>He |
:<sup>1</sup>H + <sup>16</sup>O → <sup>13</sup>N + <sup>4</sup>He |
||
The proton must be accelerated to |
The proton must be accelerated to ([[kinetic energy]] of) >5.55 MeV. |
||
The reaction is [[endothermic]] (i.e. the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass). This is one reason why the proton needs to carry extra [[energy]] to induce the [[nuclear reaction]]. |
The reaction is [[endothermic]] (i.e. the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass). This is one reason why the proton needs to carry extra [[energy]] to induce the [[nuclear reaction]]. |
||
| Line 50: | Line 52: | ||
:<math>K =(1+m/M) |E|</math> |
:<math>K =(1+m/M) |E|</math> |
||
The presence of ethanol (at a concentration of ~5mM/litre) in aqueous solution allows the convenient formation of ammonia as nitrogen-13 is produced. Other routes of producing <sup>13</sup>N-labelled ammonia exist, some of which facilitie co-generation of other light radionuclides for diagnostic imaging.<ref>{{cite web | last=Biricova | first=Veronika | last2=Kuruc | first2= Jozef | title=Synthesis of the radiopharmaceuticals for positron emission tomography | url=https://www.osti.gov/etdeweb/servlets/purl/20895812 | date=2007 | publisher=U.S. Department of Energy, Office of Scientific and Technical Information | access-date=4 August 2022}}</ref><ref>{{cite journal | journal=EJNMMI Radiopharm Chem. | volume=5 | issue=11 | pages= | doi=10.1186/s41181-020-00097-7 | date=13 May 2020 | pmid=32405797 | access-date=4 August 2022 | last1=Yokell | first1=Daniel L. | last2=Rice |first2=Peter A. | last3=Neelamegam | first3=Ramesh | last4=El Fakhri | first4=Georges | title=Development, validation and regulatory acceptance of improved purification and simplified quality control of [<sup>13</sup>N] Ammonia | url=https://pubmed.ncbi.nlm.nih.gov/32405797/}}</ref> |
|||
[[File:CNO Cycle.svg|300px|right|thumb|The N-13 role in the CNO cycle.]] |
[[File:CNO Cycle.svg|300px|right|thumb|The N-13 role in the CNO cycle.]] |
||
Revision as of 04:56, 4 August 2022
It has been suggested that this article be merged into Isotopes of nitrogen. (Discuss) Proposed since January 2022. |
| General | |
|---|---|
| Symbol | 13N |
| Names | nitrogen-13, 13N, N-13 |
| Protons (Z) | 7 |
| Neutrons (N) | 6 |
| Nuclide data | |
| Half-life (t1/2) | 9.97 min |
| Parent isotopes | 13O (β+) |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β+ | 1.2003 |
| Isotopes of nitrogen Complete table of nuclides | |
Nitrogen-13 (13N) is a radioisotope of nitrogen used in positron emission tomography (PET). It has a half-life of a little under ten minutes, so it must be made at the PET site. A cyclotron may be used for this purpose.
Nitrogen-13 is used to tag ammonia molecules for PET myocardial perfusion imaging.
Production
Nitrogen-13 is used in medical PET imaging in the form of 13N-labelled ammonia. It can be produced with a medical cyclotron, using a target of pure water with a trace amount of ethanol. The reactants are oxygen-16 and a proton, and the products are nitrogen-13 and an alpha particle (helium-4).
- 1H + 16O → 13N + 4He
The proton must be accelerated to (kinetic energy of) >5.55 MeV.
The reaction is endothermic (i.e. the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass). This is one reason why the proton needs to carry extra energy to induce the nuclear reaction.
The energy difference is actually 5.22 MeV, but if the proton only supplied this energy the reactants would be formed with no kinetic energy. As momentum must be conserved, the true energy that needs to be supplied by the proton is given by:
The presence of ethanol (at a concentration of ~5mM/litre) in aqueous solution allows the convenient formation of ammonia as nitrogen-13 is produced. Other routes of producing 13N-labelled ammonia exist, some of which facilitie co-generation of other light radionuclides for diagnostic imaging.[1][2]
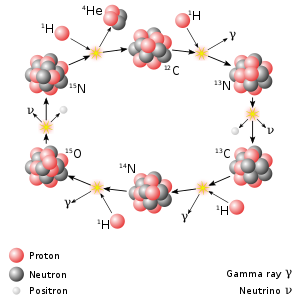
Nitrogen-13 plays a significant role in the CNO cycle, which is the dominant source of energy in stars heavier than the Sun.[3]
Lightning may have a role in the production of nitrogen-13.[4][5]
External links
- PET site of the University of Melbourne
References
- ^ Biricova, Veronika; Kuruc, Jozef (2007). "Synthesis of the radiopharmaceuticals for positron emission tomography". U.S. Department of Energy, Office of Scientific and Technical Information. Retrieved 4 August 2022.
- ^ Yokell, Daniel L.; Rice, Peter A.; Neelamegam, Ramesh; El Fakhri, Georges (13 May 2020). "Development, validation and regulatory acceptance of improved purification and simplified quality control of [13N] Ammonia". EJNMMI Radiopharm Chem. 5 (11). doi:10.1186/s41181-020-00097-7. PMID 32405797. Retrieved 4 August 2022.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Phillips, A.C. (1994). The Physics of Stars. John Wiley & Sons. ISBN 0-471-94057-7.
- ^ "Lightning, with a chance of antimatter". Phys.org. ScienceX. November 22, 2017. Retrieved November 24, 2017.
The gamma rays emitted in lightning have enough energy to knock a neutron out of atmospheric nitrogen
- ^ Castelvecchi, Davide (November 22, 2017). "Lightning makes new isotopes". Nature. doi:10.1038/nature.2017.23033. Retrieved November 29, 2017.

