2,5-Dimethylfuran: Difference between revisions
Vegaswikian (talk | contribs) m →References: Format |
Rifleman 82 (talk | contribs) →Toxicology: fix |
||
| Line 99: | Line 99: | ||
2,5-Dimethylfuran plays a role in the mechanism for the [[neurotoxicity]] of hexane in humans. Together with [[hexane-2,5-dione]] and 4,5-dihydroxy-2-hexanone, it is one of the main [[metabolite]]s of [[hexane]].<ref>Peter Arlien-Søborg. ''Solvent Neurotoxicity''. CRC Press, 1992. ISBN 0849362342. [http://books.google.com/books?doi=FC5xIyZqPKIC&pg=RA3-PA212&ots=clFo1K-pB-&dq=2,5-Dimethylfuran&as_brr=3&sig=eddaKIeD7FmzpupK_xKwJvC3gK0#PRA3-PA212,M1]</ref> |
2,5-Dimethylfuran plays a role in the mechanism for the [[neurotoxicity]] of hexane in humans. Together with [[hexane-2,5-dione]] and 4,5-dihydroxy-2-hexanone, it is one of the main [[metabolite]]s of [[hexane]].<ref>Peter Arlien-Søborg. ''Solvent Neurotoxicity''. CRC Press, 1992. ISBN 0849362342. [http://books.google.com/books?doi=FC5xIyZqPKIC&pg=RA3-PA212&ots=clFo1K-pB-&dq=2,5-Dimethylfuran&as_brr=3&sig=eddaKIeD7FmzpupK_xKwJvC3gK0#PRA3-PA212,M1]</ref> |
||
2,5-Dimethylfuran has been identified as one of the components of [[cigar]] [[smoke]] with low cilatoxicity (ability to adversely affect the [[cilia]] in the [[respiratory tract]] that are responsible for removing foreign particles).<ref>Donald Shopland. ''Cigars: Health Effects and Trends''. DIANE Publishing, 1998. [http://books.google.com/books?id=5KVvx8kNhXYC&pg=PA88&ots=7peGM2UGhA&dq=2,5-Dimethylfuran&as_brr=1&sig=Jrp4Eo5t4neA-j-iM06WUp51RTo#PPA88,M1]</ref> Its blood concentration can be used as a [[biomarker]] for [[smoking]].<ref> |
2,5-Dimethylfuran has been identified as one of the components of [[cigar]] [[smoke]] with low cilatoxicity (ability to adversely affect the [[cilia]] in the [[respiratory tract]] that are responsible for removing foreign particles).<ref>Donald Shopland. ''Cigars: Health Effects and Trends''. DIANE Publishing, 1998. [http://books.google.com/books?id=5KVvx8kNhXYC&pg=PA88&ots=7peGM2UGhA&dq=2,5-Dimethylfuran&as_brr=1&sig=Jrp4Eo5t4neA-j-iM06WUp51RTo#PPA88,M1]</ref> Its blood concentration can be used as a [[biomarker]] for [[smoking]].<ref>{{cite journal | doi = 10.1007/BF00381629}}</ref> |
||
==References== |
==References== |
||
Revision as of 16:17, 10 July 2010

| |
| |
| Names | |
|---|---|
| IUPAC name
2,5-Dimethylfuran
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.009.923 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H8O | |
| Molar mass | 96.13 |
| Appearance | Liquid |
| Density | 0.9 g/mL |
| Melting point | −62 °C |
| Boiling point | 92–94 °C |
| Insoluble | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very flammable, harmful |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
A derivative of furan, 2,5-dimethylfuran is a heterocyclic compound with the formula (CH3)2C4H2O. While it may be abbreviated DMF, it should not be confused with dimethylformamide. This simple compound is a potential biofuel, being derivable from cellulose.
Production
Fructose can be converted into 2,5-dimethylfuran in a catalytic biomass-to-liquid process. The conversion of fructose to DMF proceeds via hydroxymethylfurfural.[1][2]
Fructose is obtainable from glucose, a building block in cellulose.[3][4]
Potential as a biofuel
DMF has a number of attractions as a biofuel. It has an energy density 40% greater than ethanol, making it comparable to gasoline (petrol). It is also chemically stable and, being insoluble in water, does not absorb moisture from the atmosphere. Evaporating dimethylfuran during the production process also requires around one third less energy than the evaporation of ethanol,[1][5] although it has a boiling point some 14 °C higher, at 92 °C, compared to 78 °C for ethanol.
The ability to efficiently and rapidly produce dimethylfuran from fructose, found in fruit and some root vegetables, or from glucose, which can be derived from starch and cellulose - all widely available in nature - is likely to add to the attraction of dimethylfuran once safety issues have been examined. Bioethanol and biodiesel are currently the leading liquid biofuels.
Other uses
2,5-Dimethylfuran serves as a scavenger for singlet oxygen, a property which has been exploited for the determination of singlet oxygen in natural waters. The mechanism involves a Diels-Alder reaction followed by hydrolysis, ultimately leading to diacetylethylene and hydrogen peroxide as products. More recently, furfuryl alcohol has been used for the same purpose.[6]
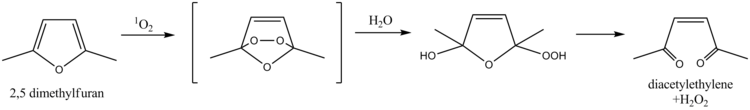
This compound has also been proposed as an internal standard for NMR spectroscopy. 2,5-Dimethylfuran has two singlets in its NMR spectrum at δ 2.2 and 5.8; the singlets give reliable integrations, while the positions of the peaks do not interfere with most analytes. The compound also has an appropriate boiling point of 92 °C which prevents evaporative losses, yet is easily removed.[7]
Role in food chemistry
2,5-Dimethylfuran can be formed from the thermal degradation of sugars, and has been identified in trace amounts as a component of caramelized sugars.[8]
Toxicology
2,5-Dimethylfuran plays a role in the mechanism for the neurotoxicity of hexane in humans. Together with hexane-2,5-dione and 4,5-dihydroxy-2-hexanone, it is one of the main metabolites of hexane.[9]
2,5-Dimethylfuran has been identified as one of the components of cigar smoke with low cilatoxicity (ability to adversely affect the cilia in the respiratory tract that are responsible for removing foreign particles).[10] Its blood concentration can be used as a biomarker for smoking.[11]
References
- ^ a b Yuriy Román-Leshkov, Christopher J. Barrett, Zhen Y. Liu & James A. Dumesic (2007). "Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates". Nature. 447 (7147): 982. doi:10.1038/nature05923. PMID 17581580.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Matt McGrath (2007-06-21). "Fruit could make 'powerful fuel'". BBC News. Retrieved 2007-06-22.
- ^ Haibo Zhao, Johnathan E. Holladay, Heather Brown, Z. Conrad Zhang (June 15, 2007). "Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural". Science. 316 (5831): 1597–1600. doi:10.1126/science.1141199. PMID 17569858.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Plastic that grows on trees" (press release). Pacific Northwest National Laboratory. 2007-06-21. Retrieved 2007-06-22.
- ^ James Beal (2007-06-20). "Engineers develop higher-energy liquid-transportation fuel from sugar" (press release). University of Wisconsin-Madison. Retrieved 2007-06-22.
- ^ Patrick L. Brezonik. Chemical Kinetics and Process Dynamics in Aquatic Systems. CRC Press, 1994, p. 671. [1]
- ^ S. W. Gerritz and A. M. Sefler (2000). "2,5-Dimethylfuran (DMFu): An Internal Standard for the "Traceless" Quantitation of Unknown Samples via 1H NMR". J. Comb. Chem. 2 (1): 39–41. doi:10.1021/cc990041v.
- ^ W.D. Powrie, C.H. Wu, V.P. Molund (1986). "Browning Reaction Systems as Sources of Mutagens and Antimutagens". Environmental Health Perspectives. 67. Environmental Health Perspectives, Vol. 67: 47–54. doi:10.2307/3430317.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peter Arlien-Søborg. Solvent Neurotoxicity. CRC Press, 1992. ISBN 0849362342. [2]
- ^ Donald Shopland. Cigars: Health Effects and Trends. DIANE Publishing, 1998. [3]
- ^ . doi:10.1007/BF00381629.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)
