Ε-Viniferin: Difference between revisions
Content deleted Content added
m r2.7.3) (Robot: Adding fa:اپسیلون-وینیفرین |
use cite templates |
||
| Line 10: | Line 10: | ||
| ImageCaption = (-)-Trans-epsilon-viniferin |
| ImageCaption = (-)-Trans-epsilon-viniferin |
||
| IUPACName = 5-[(2''R'',3''R'')-6-Hydroxy-2-(4-hydroxyphenyl)-4-[(''E'')-2-(4-hydroxyphenyl)ethenyl]-2,3-dihydro-1-benzofuran-3-yl]benzene-1,3-diol |
| IUPACName = 5-[(2''R'',3''R'')-6-Hydroxy-2-(4-hydroxyphenyl)-4-[(''E'')-2-(4-hydroxyphenyl)ethenyl]-2,3-dihydro-1-benzofuran-3-yl]benzene-1,3-diol |
||
| OtherNames = Viniferin<br>''epsilon''-Viniferin<br>(-)-''epsilon''-Viniferin<br>(-)-(E)-epsilon-viniferin<br>trans-ε-viniferin<br>(-)-Trans-epsilon-viniferin<ref> |
| OtherNames = Viniferin<br>''epsilon''-Viniferin<br>(-)-''epsilon''-Viniferin<br>(-)-(E)-epsilon-viniferin<br>trans-ε-viniferin<br>(-)-Trans-epsilon-viniferin<ref>{{Cite doi|10.1016/j.ejphar.2006.06.005|noedit}}</ref><br><nowiki>Iso-[epsilon]-viniferin</nowiki><ref>{{Cite PMID|17691204|noedit}}</ref><br>cis-ε-viniferin<br>Cis-epsilon-viniferin<ref name=Kim>{{Cite doi|10.1271/bbb.66.1990|noedit}}</ref> |
||
|Section1= {{Chembox Identifiers |
|Section1= {{Chembox Identifiers |
||
| CASNo_Ref = {{cascite|changed|??}} |
| CASNo_Ref = {{cascite|changed|??}} |
||
| Line 46: | Line 46: | ||
'''ε-Viniferin''' is a naturally occurring [[natural phenol|phenol]], belonging to the [[stilbenoid]]s family. It is a [[resveratrol]] [[Dimer (chemistry)|dimer]]. |
'''ε-Viniferin''' is a naturally occurring [[natural phenol|phenol]], belonging to the [[stilbenoid]]s family. It is a [[resveratrol]] [[Dimer (chemistry)|dimer]]. |
||
It is found in ''[[Vitis vinifera]]''<ref> |
It is found in ''[[Vitis vinifera]]''<ref>{{Cite doi|10.1021/jf010676t|noedit}}</ref> grapevines,<ref>{{Cite doi|10.1007/BF02124034|noedit}}</ref> in [[wine]]s,<ref>{{Cite pmid|15998130}}</ref> in the Oriental medicinal plant ''[[Vitis coignetiae]]'' and in the stem bark of ''[[Dryobalanops aromatica]]''.<ref>{{Cite doi|10.1016/j.fitote.2011.02.006|noedit}}</ref> |
||
<br>Cis-epsilon-viniferin can be found in ''[[Paeonia lactiflora]]''.<ref name=Kim/> |
<br>Cis-epsilon-viniferin can be found in ''[[Paeonia lactiflora]]''.<ref name=Kim/> |
||
It shows a human [[cytochrome P450]] enzymes inhition activity.<ref> |
It shows a human [[cytochrome P450]] enzymes inhition activity.<ref>{{Cite doi|10.1016/S0024-3205(03)00420-X|noedit}}</ref> |
||
== Glycosides == |
== Glycosides == |
||
Revision as of 20:27, 22 May 2012
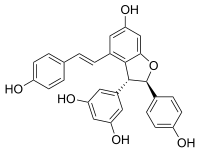 (-)-Trans-epsilon-viniferin
| |
| Names | |
|---|---|
| IUPAC name
5-[(2R,3R)-6-Hydroxy-2-(4-hydroxyphenyl)-4-[(E)-2-(4-hydroxyphenyl)ethenyl]-2,3-dihydro-1-benzofuran-3-yl]benzene-1,3-diol
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C28H22O6 | |
| Molar mass | 454.47 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.
It is found in Vitis vinifera[4] grapevines,[5] in wines,[6] in the Oriental medicinal plant Vitis coignetiae and in the stem bark of Dryobalanops aromatica.[7]
Cis-epsilon-viniferin can be found in Paeonia lactiflora.[3]
It shows a human cytochrome P450 enzymes inhition activity.[8]
Glycosides
Diptoindonesin A is a C-glucoside of ε-viniferin.
See also
References
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.ejphar.2006.06.005, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.ejphar.2006.06.005instead. - ^ Template:Cite PMID
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1271/bbb.66.1990, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1271/bbb.66.1990instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jf010676t, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jf010676tinstead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF02124034, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF02124034instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15998130, please use {{cite journal}} with
|pmid=15998130instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.fitote.2011.02.006, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.fitote.2011.02.006instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0024-3205(03)00420-X, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0024-3205(03)00420-Xinstead.
