Carbide-derived carbon: Difference between revisions
m quick formatting fixes |
|||
| Line 1: | Line 1: | ||
| ⚫ | |||
'''Carbide-Derived Carbon''' (CDC), also known as '''Tunable Nanoporous Carbon''', is the common term for carbon materials derived from |
|||
| ⚫ | |||
[[carbide]] precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as [[ |
'''Carbide-derived carbon''' (CDC), also known as '''tunable nanoporous carbon''', is the common term for carbon materials derived from [[carbide]] precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as [[MAX phases]] (e.g., Ti<sub>2</sub>AlC, Ti<sub>3</sub>SiC<sub>2</sub>).<ref name="a1" /><ref name="a2" /><ref name="a3" /><ref name="a4" /> CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C <ref name="a5" /><ref name="a6" /><ref name="a7" />. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp<sup>2</sup>- to sp<sup>3</sup>-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: [[Microporous_material|micro-]] and [[Mesoporous_material|mesoporous]] carbon, amorphous carbon, [[Carbon_nanotube|carbon nanotubes]], onion-like carbon, [[Nanodiamond|nanocrystalline diamond]], [[graphene]], and [[graphite]]<ref name="a1" />. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m<sup>2</sup>/g)<ref name="a8" />. By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy<ref name="a9" />. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO<sub>2</sub>) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization. |
||
==History== |
==History== |
||
The process of using high-temperature chlorine treatment for production of SiCl<sub>4</sub> by etching of metal carbides was first patented in 1918 by Otis Hutchins<ref name="a10" />, with the process further optimized for higher yields in 1956<ref name="a11" />. The solid porous carbon product was initially regarded as a waste byproduct until its properties and potential applications were investigated in more detail in 1959 by Walter Mohun<ref name="a12" />. Research was carried out in the 1960-1980s mostly by Russian scientists on the synthesis of CDC via halogen treatment<ref name="a13" /> |
The process of using high-temperature chlorine treatment for production of SiCl<sub>4</sub> by etching of metal carbides was first patented in 1918 by Otis Hutchins<ref name="a10" />, with the process further optimized for higher yields in 1956<ref name="a11" />. The solid porous carbon product was initially regarded as a waste byproduct until its properties and potential applications were investigated in more detail in 1959 by Walter Mohun<ref name="a12" />. Research was carried out in the 1960-1980s mostly by Russian scientists on the synthesis of CDC via halogen treatment<ref name="a13" /><ref name="a14" />, while hydrothermal treatment was explored as an alternative route to derive CDCs in the 1990s<ref name="a15" />. Most recently, research activities have centered on optimized CDC synthesis and nanoengineered CDC precursors. |
||
==Nomenclature== |
==Nomenclature== |
||
| Line 10: | Line 10: | ||
==Synthesis== |
==Synthesis== |
||
CDCs have been synthesized using several chemical and physical synthesis methods. Most commonly, dry chlorine treatment is used to selectively etch metal or metalloid atoms from the carbide precursor lattice<ref name="a1" />. The term “chlorine treatment” is to be preferred over [[halogenation|chlorination]] as the chlorinated product, metal chloride, is the discarded byproduct and the carbon itself remains largely unreacted. This method is implemented for commercial production of CDC by Skeleton in Estonia and Y-Carbon in the USA. The vacuum decomposition of SiC wafers (i.e., single crystals) is used for the synthesis of epitaxial graphene with homogeneous areas in the cm<sup>2</sup> range<ref name="a16" />. Hydrothermal etching has also been used for synthesis of SiC-CDC which yielded a route for porous carbon films and nanodiamond synthesis<ref name="a17" /> |
CDCs have been synthesized using several chemical and physical synthesis methods. Most commonly, dry chlorine treatment is used to selectively etch metal or metalloid atoms from the carbide precursor lattice<ref name="a1" />. The term “chlorine treatment” is to be preferred over [[halogenation|chlorination]] as the chlorinated product, metal chloride, is the discarded byproduct and the carbon itself remains largely unreacted. This method is implemented for commercial production of CDC by Skeleton in Estonia and Y-Carbon in the USA. The vacuum decomposition of SiC wafers (i.e., single crystals) is used for the synthesis of epitaxial graphene with homogeneous areas in the cm<sup>2</sup> range<ref name="a16" />. Hydrothermal etching has also been used for synthesis of SiC-CDC which yielded a route for porous carbon films and nanodiamond synthesis<ref name="a17" /><ref name="a18" />. |
||
[[Image:Figure2CDC2.jpg|thumb|right|Schematic of chlorine etching of to produce a porous carbon structure.]] |
[[Image:Figure2CDC2.jpg|thumb|right|Schematic of chlorine etching of to produce a porous carbon structure.]] |
||
| Line 16: | Line 16: | ||
The most common method for producing porous carbide-derived carbons involves high-temperature etching with halogens, most commonly chlorine gas. The following generic equation describes the reaction of a metal chloride with chlorine gas (M: Si, Ti, V; similar equations can be written for other CDC precursors): |
The most common method for producing porous carbide-derived carbons involves high-temperature etching with halogens, most commonly chlorine gas. The following generic equation describes the reaction of a metal chloride with chlorine gas (M: Si, Ti, V; similar equations can be written for other CDC precursors): |
||
MC(solid) + |
:MC(solid) + 2 Cl<sub>2</sub>(gas) → MCl<sub>4</sub>(gas) + C(solid) |
||
Halogen treatment at temperatures between 200 and 1000°C has been shown to yield mostly disordered porous carbons with a porosity between 50 and ~80 vol% depending on the precursor. Temperatures above 1000°C result in predominantly graphitic carbon and an observed shrinkage of the material due to graphitization. |
Halogen treatment at temperatures between 200 and 1000 °C has been shown to yield mostly disordered porous carbons with a porosity between 50 and ~80 vol% depending on the precursor. Temperatures above 1000 °C result in predominantly graphitic carbon and an observed shrinkage of the material due to graphitization. |
||
[[Image:Figure3CDC2.jpg|thumb|right|Different bulk porosity of CDCs derived from different carbide precursors.]] |
[[Image:Figure3CDC2.jpg|thumb|right|Different bulk porosity of CDCs derived from different carbide precursors.]] |
||
The linear growth rate of the solid carbon product phase suggests a reaction-driven kinetic mechanism, but the kinetics become diffusion-limited for thicker films or larger particles. A high mass transport condition (high gas flow rates) facilitates the removal of the chloride and shifts the reaction equilibrium towards the CDC product. Chlorine treatment has successfully been employed for CDC synthesis from a variety of carbide precursors, including SiC, TiC, B<sub>4</sub>C, BaC<sub>2</sub>, CaC<sub>2</sub>, Cr<sub>3</sub>C<sub>2</sub>, Fe<sub>3</sub>C, Mo<sub>2</sub>C, Al<sub>4</sub>C<sub>3</sub>, Nb<sub>2</sub>C, SrC<sub>2</sub>, Ta<sub>2</sub>C, VC, WC, W<sub>2</sub>C, ZrC, ternary carbides such as Ti<sub>2</sub>AlC, Ti<sub>3</sub>AlC<sub>2</sub>, and Ti<sub>3</sub>SiC<sub>2</sub>, and carbonitrides such as Ti<sub>2</sub>AlC<sub>0.5</sub>N<sub>0.5</sub>. |
The linear growth rate of the solid carbon product phase suggests a reaction-driven kinetic mechanism, but the kinetics become diffusion-limited for thicker films or larger particles. A high mass transport condition (high gas flow rates) facilitates the removal of the chloride and shifts the reaction equilibrium towards the CDC product. Chlorine treatment has successfully been employed for CDC synthesis from a variety of carbide precursors, including SiC, TiC, B<sub>4</sub>C, BaC<sub>2</sub>, CaC<sub>2</sub>, Cr<sub>3</sub>C<sub>2</sub>, Fe<sub>3</sub>C, Mo<sub>2</sub>C, Al<sub>4</sub>C<sub>3</sub>, Nb<sub>2</sub>C, SrC<sub>2</sub>, Ta<sub>2</sub>C, VC, WC, W<sub>2</sub>C, ZrC, ternary carbides such as Ti<sub>2</sub>AlC, Ti<sub>3</sub>AlC<sub>2</sub>, and Ti<sub>3</sub>SiC<sub>2</sub>, and carbonitrides such as Ti<sub>2</sub>AlC<sub>0.5</sub>N<sub>0.5</sub>. |
||
Most produced CDCs exhibit a prevalence of micropores (< 2 nm) and mesopores (between 2 and 50 nm), with specific distributions affected by carbide precursor and synthesis conditions<ref name="a19" />. Hierarchic porosity can be achieved by using polymer-derived ceramics with or without utilizing a templating method<ref name="a20" />. Templating yields an ordered array of mesopores in addition to the disordered network of micropores. |
Most produced CDCs exhibit a prevalence of micropores (< 2 nm) and mesopores (between 2 and 50 nm), with specific distributions affected by carbide precursor and synthesis conditions<ref name="a19" />. Hierarchic porosity can be achieved by using polymer-derived ceramics with or without utilizing a templating method<ref name="a20" />. Templating yields an ordered array of mesopores in addition to the disordered network of micropores. |
||
It has been shown that the initial crystal structure of the carbide is the primary factor affecting the CDC porosity, especially for low-temperature chlorine treatment. In general, a larger spacing between carbon atoms in the lattice correlates with an increase in the average pore diameter<ref name="a2" /> |
It has been shown that the initial crystal structure of the carbide is the primary factor affecting the CDC porosity, especially for low-temperature chlorine treatment. In general, a larger spacing between carbon atoms in the lattice correlates with an increase in the average pore diameter<ref name="a2" /><ref name="a21" />. As the synthesis temperature increases, the average pore diameter increases, while the pore size distribution becomes broader<ref name="a9" />. The overall shape and size of the carbide precursor, however, is largely maintained and CDC formation is usually referred to as a conformal process<ref name="a19" />. |
||
[[Image:Figure4CDC.jpg|thumb|right|Pore size distributions for different carbide precursors.]] |
[[Image:Figure4CDC.jpg|thumb|right|Pore size distributions for different carbide precursors.]] |
||
===Vacuum |
===Vacuum decomposition=== |
||
''Main |
''Main article: ''[[Graphene#Epitaxial_growth_on_silicon_carbide|''Epitaxial graphene'']]<br /> |
||
Metal or metalloid atoms from carbides can selectively be extracted at high temperatures (usually above 1200°C) under vacuum. The underlying mechanism is incongruent decomposition of carbides, using the high melting point of carbon compared to corresponding carbide metals that melt and eventually evaporate away, leaving the carbon behind<ref name="a22" />. |
Metal or metalloid atoms from carbides can selectively be extracted at high temperatures (usually above 1200 °C) under vacuum. The underlying mechanism is incongruent decomposition of carbides, using the high melting point of carbon compared to corresponding carbide metals that melt and eventually evaporate away, leaving the carbon behind<ref name="a22" />. |
||
Like halogen treatment, vacuum decomposition is a conformal process<ref name="a19" />. The resulting carbon structures are, as a result of the higher temperatures, more ordered, and carbon nanotubes and graphene can be obtained. In particular, vertically aligned carbon nanotubes films of high tube density have been reported for vacuum decomposition of SiC<ref name="a23" />. The high tube density translates into a high elastic modulus and high buckling resistance which is of particular interest for mechanical and tribological applications<ref name="a24" />. |
Like halogen treatment, vacuum decomposition is a conformal process<ref name="a19" />. The resulting carbon structures are, as a result of the higher temperatures, more ordered, and carbon nanotubes and graphene can be obtained. In particular, vertically aligned carbon nanotubes films of high tube density have been reported for vacuum decomposition of SiC<ref name="a23" />. The high tube density translates into a high elastic modulus and high buckling resistance which is of particular interest for mechanical and tribological applications<ref name="a24" />. |
||
While carbon nanotube formation occurs when trace oxygen amounts are present, very high vacuum conditions (approaching 10<sup> |
While carbon nanotube formation occurs when trace oxygen amounts are present, very high vacuum conditions (approaching 10<sup>−8</sup>-10<sup>−10</sup> torr) result in the formation of graphene sheets. If the conditions are maintained, graphene transitions into bulk graphite. In particular, by vacuum annealing silicon carbide single crystals (wafers) at 1200–1500 °C<ref name="a16" />, metal/metalloid atoms are selectively removed and a layer of 1–3 layer graphene (depending on the treatment time) is formed, undergoing a conformal transformation of 3 layers of silicon carbide into one monolayer of graphene <ref name="a25" />. Also, graphene formation occurs prefentially on the Si-face of the 6H-SiC crystals, while nanotube growth is favored on the c-face of SiC<ref name="a23" />. |
||
===Hydrothermal |
===Hydrothermal decomposition=== |
||
The removal of metal atoms from carbides has been reported at high temperatures ( |
The removal of metal atoms from carbides has been reported at high temperatures (300–1000 °C) and pressures (2-200 MPa). The following reactions are possible between metal carbides and water: |
||
(a) <sup>x</sup>/<sub>2</sub>•MC + x•H<sub>2</sub>O → M<sub>x/2</sub>O<sub>x</sub> + <sup>x</sup>/<sub>2</sub>•CH<sub>4</sub><br /> |
(a) <sup>x</sup>/<sub>2</sub>•MC + x•H<sub>2</sub>O → M<sub>x/2</sub>O<sub>x</sub> + <sup>x</sup>/<sub>2</sub>•CH<sub>4</sub><br /> |
||
| Line 46: | Line 46: | ||
(d) MC + x•H<sub>2</sub>O → MO<sub>x</sub> + C + x•H<sub>2</sub> |
(d) MC + x•H<sub>2</sub>O → MO<sub>x</sub> + C + x•H<sub>2</sub> |
||
Only reaction (d) yields solid carbon. The favorability of the formation of carbon-containing gases increases with higher pressure (decreasing solid carbon yield) and decreases at higher temperatures (increasing the carbon yield). The ability to produce a usable porous carbon material is dependent on the solubility of the formed metal oxide (such as SiO<sub>2</sub>) in supercritical water. Hydrothermal carbon formation has been reported for SiC, TiC, WC, TaC, and NbC. Insolubility of metal oxides, for example TiO<sub>2</sub>, is a significant complication for certain metal carbides (e.g., Ti<sub>3</sub>SiC<sub>2</sub>) |
Only reaction (d) yields solid carbon. The favorability of the formation of carbon-containing gases increases with higher pressure (decreasing solid carbon yield) and decreases at higher temperatures (increasing the carbon yield). The ability to produce a usable porous carbon material is dependent on the solubility of the formed metal oxide (such as SiO<sub>2</sub>) in supercritical water. Hydrothermal carbon formation has been reported for SiC, TiC, WC, TaC, and NbC. Insolubility of metal oxides, for example TiO<sub>2</sub>, is a significant complication for certain metal carbides (e.g., Ti<sub>3</sub>SiC<sub>2</sub>).<ref name="a19" /><ref name="a26" /> |
||
==Applications== |
==Applications== |
||
===Energy |
===Energy storage=== |
||
{{See also|Electric double-layer capacitor}} |
{{See also|Electric double-layer capacitor|Capa vehicle}} |
||
| ⚫ | One application of carbide-derived carbons is as active material in electrodes for electric double layer capacitors which have become commonly known as supercapacitors or ultracapacitors. This is motivated by their good electrical conductivity combined with high surface area<ref name="a27" />, large micropore volume<ref name="a21" />, and pore size control<ref name="a28" /> that enable to match the porosity metrics of the porous carbon electrode to a certain electrolyte<ref name="a29" />. In particular, when the pore size approaches the size of the (desolvated) ion in the electrolyte, there is a significant increase in the capacitance. The electrically conductive carbon material minimizes resistance losses in supercapacitor devices and enhances charge screening and confinement<ref name="a30" />, maximizing the packing density and subsequent charge storage capacity of microporous CDC electrodes<ref name="a31" /><ref name="a32" /><ref name="a33" />. |
||
{{See also|Capa vehicle}} |
|||
| ⚫ | One application of carbide-derived carbons is as active material in electrodes for electric double layer capacitors which have become commonly known as supercapacitors or ultracapacitors. This is motivated by their good electrical conductivity combined with high surface area<ref name="a27" />, large micropore volume<ref name="a21" />, and pore size control<ref name="a28" /> that enable to match the porosity metrics of the porous carbon electrode to a certain electrolyte<ref name="a29" />. In particular, when the pore size approaches the size of the (desolvated) ion in the electrolyte, there is a significant increase in the capacitance. The electrically conductive carbon material minimizes resistance losses in supercapacitor devices and enhances charge screening and confinement<ref name="a30" />, maximizing the packing density and subsequent charge storage capacity of microporous CDC electrodes<ref name="a31" /> |
||
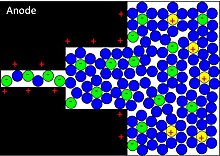
[[Image:Figure5CDC.jpg|thumb|right|Confinement of solvated ions in pores, such as those present in CDCs. As the pore size approaches the size of the solvation shell, the solvent molecules are removed, resulting in larger ionic packing density and increased charge storage capability.]] |
[[Image:Figure5CDC.jpg|thumb|right|Confinement of solvated ions in pores, such as those present in CDCs. As the pore size approaches the size of the solvation shell, the solvent molecules are removed, resulting in larger ionic packing density and increased charge storage capability.]] |
||
CDC electrodes have been shown to yield a gravimetric capacitance of up to 190 F/g in aqueous electrolytes and 180 F/g in organic electrolytes<ref name="a29" />. The highest capacitance values are observed for matching ion/pore systems, which allow high-density packing of ions in pores in superionic states<ref name="a34" />. However, small pores, especially when combined with an overall large particle diameter, impose an additional diffusion limitation on the ion mobility during charge/discharge cycling. The prevalence of mesopores in the CDC structure allows for more ions to move past each other during charging and discharging, allowing for faster scan rates and improved rate handling abilities<ref name="a35" />. Conversely, by implementing nanoparticle carbide precursors, shorter pore channels allow for higher electrolyte mobility, resulting in faster charge/discharge rates and higher power densities <ref name="a36" />. |
CDC electrodes have been shown to yield a gravimetric capacitance of up to 190 F/g in aqueous electrolytes and 180 F/g in organic electrolytes<ref name="a29" />. The highest capacitance values are observed for matching ion/pore systems, which allow high-density packing of ions in pores in superionic states<ref name="a34" />. However, small pores, especially when combined with an overall large particle diameter, impose an additional diffusion limitation on the ion mobility during charge/discharge cycling. The prevalence of mesopores in the CDC structure allows for more ions to move past each other during charging and discharging, allowing for faster scan rates and improved rate handling abilities<ref name="a35" />. Conversely, by implementing nanoparticle carbide precursors, shorter pore channels allow for higher electrolyte mobility, resulting in faster charge/discharge rates and higher power densities <ref name="a36" />. |
||
===Gas storage and |
===Gas storage and carbon dioxide capturing=== |
||
TiC-CDC activated with KOH or CO<sub>2</sub> has been shown to store up to 21 wt.% of methane at 25°C at high pressure. In particular, CDCs with subnanometer pores in the 0. |
TiC-CDC activated with KOH or CO<sub>2</sub> has been shown to store up to 21 wt.% of methane at 25 °C at high pressure. In particular, CDCs with subnanometer pores in the 0.50–0.88 nm diameter range have shown to store up to 7.1 mol CO<sub>2</sub>/kg at 1 bar and 0 °C.<ref name="a37" /> CDCs have also been shown to store up to 3 wt.% hydrogen at 60 bar and −196 °C, with additional increases possible as a result of chemical or physical activation of the CDC materials. SiOC-CDC with large subnanometer pore volumes are able to store over 5.5 wt.% hydrogen at 60 bar and −196 °C, almost reaching the goal of the US Department of Energy of 6 wt.% storage density for automotive applications. Methane storage densities of over 21.5 wt.% can be achieved for this material at those conditions. In particular, a predominance of pores with subnanometer diameters and large pore volumes are instrumental towards increasing storage densities<ref name="a38" />. |
||
===Tribological coatings=== |
===Tribological coatings=== |
||
| Line 64: | Line 63: | ||
===Protein adsorption=== |
===Protein adsorption=== |
||
Carbide-derived carbons with a mesoporous structure have been shown to remove large molecules from biofluids. As other carbons, CDCs possess good biocompatibility<ref name="a41" />. CDCs have been demonstrated to remove cytokines such as TNF-alpha, IL-6, and IL-1beta from blood plasma. These are the most common receptor-binding agents released into the body during a bacterial infection that cause the primary inflammatory response during the attack and increase the potential lethality of sepsis, making their removal a very important concern<ref name="a42" />. The rates and levels of removal of above cytokines ( |
Carbide-derived carbons with a mesoporous structure have been shown to remove large molecules from biofluids. As other carbons, CDCs possess good biocompatibility<ref name="a41" />. CDCs have been demonstrated to remove cytokines such as TNF-alpha, IL-6, and IL-1beta from blood plasma. These are the most common receptor-binding agents released into the body during a bacterial infection that cause the primary inflammatory response during the attack and increase the potential lethality of sepsis, making their removal a very important concern<ref name="a42" />. The rates and levels of removal of above cytokines (85–100% removed within 30 minutes) are higher than those observed for comparable activated carbons<ref name="a42" />. |
||
===Catalyst support=== |
===Catalyst support=== |
||
| Line 72: | Line 71: | ||
===Capacitive deionization (CDI)=== |
===Capacitive deionization (CDI)=== |
||
{{See also|Capacitive deionization}} |
{{See also|Capacitive deionization}} |
||
As desalinization and purification of water is critical for obtaining deionized water for laboratory research, large-scale chemical synthesis in industry and consumer applications, the use of porous materials for this application has received particular interest. Capacitive deionization operates in a fashion with similarities to a supercapacitor. As an ion-containing water (electrolyte) is flown between two porous electrodes with an applied potential across the system, the corresponding ions assemble into a double layer in the pores of the two terminals, decreasing the ion content in the liquid exiting the purification device<ref name="a45" />. Due to the ability of carbide-derived carbons to closely match the size of ions in the electrolyte, side-by-side comparisons of desalinization devices based on CDCs and activated carbon showed a significant efficiency increase in the 1. |
As desalinization and purification of water is critical for obtaining deionized water for laboratory research, large-scale chemical synthesis in industry and consumer applications, the use of porous materials for this application has received particular interest. Capacitive deionization operates in a fashion with similarities to a supercapacitor. As an ion-containing water (electrolyte) is flown between two porous electrodes with an applied potential across the system, the corresponding ions assemble into a double layer in the pores of the two terminals, decreasing the ion content in the liquid exiting the purification device<ref name="a45" />. Due to the ability of carbide-derived carbons to closely match the size of ions in the electrolyte, side-by-side comparisons of desalinization devices based on CDCs and activated carbon showed a significant efficiency increase in the 1.2–1.4 V range compared to activated carbon<ref name="a45" />. |
||
==Commercial |
==Commercial production and applications== |
||
Having originated as the by-product of industrial metal chloride synthesis, CDC has certainly a potential for large-scale production at a moderate cost. Currently, two small companies engage in scalable production of carbide-derived carbons and their implementation in commercial products. Skeleton, which is located in Tartu, Estonia, has a diverse product line of porous carbons for supercapacitors, gas storage, and filtration applications. Y-Carbon, which is based in Bristol, Pennsylvania (United States), is also involved in the production and development of carbide-derived carbons for water desalination, special sorbents, chromatography, filtration, and electrical energy storage and generation. In addition, numerous education and research institutions worldwide are engaged in basic research of CDC structure, synthesis, or (indirectly) their application for various high-end applications. |
Having originated as the by-product of industrial metal chloride synthesis, CDC has certainly a potential for large-scale production at a moderate cost. Currently, two small companies engage in scalable production of carbide-derived carbons and their implementation in commercial products. Skeleton, which is located in Tartu, Estonia, has a diverse product line of porous carbons for supercapacitors, gas storage, and filtration applications. Y-Carbon, which is based in Bristol, Pennsylvania (United States), is also involved in the production and development of carbide-derived carbons for water desalination, special sorbents, chromatography, filtration, and electrical energy storage and generation. In addition, numerous education and research institutions worldwide are engaged in basic research of CDC structure, synthesis, or (indirectly) their application for various high-end applications. |
||
==See also== |
==See also== |
||
* [[Hydrogen storage]] |
* [[Hydrogen storage]] |
||
| Line 87: | Line 85: | ||
==References== |
==References== |
||
{{Reflist| |
{{Reflist|35em|refs= |
||
| ⚫ | |||
refs= |
|||
<ref name=" |
<ref name="a2">Kyotani, T., Chmiola, J. & Gogotsi, Y. in ''Carbon Materials for Electrochemical Energy Storage Systems'' (eds F. Beguin & E. Frackowiak) Ch. 3, 77–113 (CRC Press/Taylor and Francis, 2009).</ref> |
||
<ref name=" |
<ref name="a3">Yushin, G., Nikitin, A. & Gogotsi, Y. (2006) in ''Carbon Nanomaterials'', Y. Gogotsi (ed.) pp. 211–254, CRC Taylor & Francis ISBN 0849393868.</ref> |
||
<ref name=" |
<ref name="a4">Nikitin, A. & Gogotsi, Y. (2004) in ''Encyclopedia of Nanoscience and Nanotechnology'' Vol. 7, H.S. Nalwa (ed.) pp. 553–574, American Scientific Publishers</ref> |
||
| ⚫ | |||
<ref name="a4">Nikitin, A. & Gogotsi, Y. in ''Encyclopedia of Nanoscience and Nanotechnology'' Vol. 7 (ed H.S. Nalwa) 553-574 (American Scientific Publishers, 2004).</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | <ref name="a8">{{cite journal|author=Rose, M., Kockrick, E., Senkovska, I. & Kaskel, S. |title=High surface area carbide-derived carbon fibers produced by electrospinning of polycarbosilane precursors|journal=Carbon|volume= 48|pages= 403–407 |year=2010|doi=10.1016/j.carbon.2009.09.043|issue=2}}</ref> |
||
| ⚫ | |||
<ref name="a9">{{cite journal|author=Gogotsi, Y. et al. |title=Nanoporous carbide-derived carbon with tunable pore size|journal=Nature Materials|volume= 2|pages= 591–594 |year=2003|doi=10.1038/nmat957|pmid=12907942|issue=9}}</ref> |
|||
| ⚫ | |||
<ref name=" |
<ref name="a10">Hutchins, O. Method for the Production of Silicon Tetrachlorid. {{US patent|1271713}} (1918)</ref> |
||
<ref name=" |
<ref name="a11">Andersen, J. N. Silicon Tetrachloride Manufacture. {{US patent|2739041}} (1956)</ref> |
||
<ref name=" |
<ref name="a12">Mohun, W. A. in ''Proceedings of the Conference on Carbon'' Vol. 4 pp. 443–453 (1959)</ref> |
||
<ref name="a13">{{cite journal|author=Babkin, O. E., Ivakhnyuk, G. K., Lukin, Y. N. & Fedorov, N. F.|title= Study of structure of carbide derived carbon by XPS|journal=Zhurnal Prikladnoi Khimii|volume= 57|pages= 1719–1721 |year=1988}}</ref> |
|||
<ref name="a12">Mohun, W. A. in ''Proceedings of the Conference on Carbon'' Vol. 4 443 - 453 (1959).</ref> |
|||
<ref name=" |
<ref name="a14">{{cite journal|author=Gordeev, S. K. & Vartanova, A. V.|title= New approach for production of block microporous materials|journal=Zhurnal Prikladnoi Khimii|volume= 67|pages= 1375–1377 |year=1994}}</ref> |
||
| ⚫ | |||
<ref name="a14">Gordeev, S. K. & Vartanova, A. V. New approach for production of block microporous materials. ''Zhurnal Prikladnoi Khimii'' 67, 1375 - 1377 (1994).</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | <ref name="a18">{{cite journal|author=Kitaoka, S., Tsuji, T., Katoh, T. & Yamaguchi, Y. |title=Tribological Characteristics of SiC Ceramics in High-Temperature and High-Pressure Water|journal=Journal of the American Ceramic Society|volume= 77|pages=1851–1856 |year=1994|doi=10.1111/j.1151-2916.1994.tb07061.x|issue=7}}</ref> |
||
| ⚫ | |||
<ref name="a19">{{cite journal|author=Presser, V., Heon, M. & Gogotsi, Y. |title=Carbide-Derived Carbons-From Porous Networks to Nanotubes and Graphene|journal=Advanced Functional Materials|volume= 21|pages= 810–833 |year=2011|doi=10.1002/adfm.201002094|issue=5}}</ref> |
|||
| ⚫ | |||
<ref name="a20">{{cite journal|author=Kockrick, E. et al.|title= Ordered mesoporous carbide derived carbons for high pressure gas storage|journal=Carbon|volume= 48 |pages=1707–1717 |year=2010|doi=10.1016/j.carbon.2010.01.004|issue=6}}</ref> |
|||
| ⚫ | |||
<ref name=" |
<ref name="a21">{{cite journal|author=Arulepp, M. et al. |title=The advanced carbide-derived carbon based supercapacitor|journal=Journal of Power Sources|volume= 162|pages= 1460–1466 |year=2006|doi=10.1016/j.jpowsour.2006.08.014|issue=2}}</ref> |
||
| ⚫ | |||
<ref name="a21">Arulepp, M. et al. The advanced carbide-derived carbon based supercapacitor. ''Journal of Power Sources'' 162, 1460-1466 (2006).</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | <ref name="a26">{{cite journal|author=Hoffman, E. N., Yushin, G., El-Raghy, T., Gogotsi, Y. & Barsoum, M. W.|title= Micro and mesoporosity of carbon derived from ternary and binary metal carbides|journal=Microporous and Mesoporous Materials|volume= 112|pages= 526–532 |year=2008|doi=10.1016/j.micromeso.2007.10.033}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<ref name="a32">{{cite journal|author=Permann, L., Latt, M., Leis, J. & Arulepp, M.|title= Electrical double layer characteristics of nanoporous carbon derived from titanium carbide|journal=Electrochimica Acta|volume= 51|pages= 1274–1281 |year=2006|doi=10.1016/j.electacta.2005.06.024|issue=7}}</ref> |
|||
| ⚫ | |||
<ref name=" |
<ref name="a33">{{cite journal|author=Leis, J., Arulepp, M., Kuura, A., Latt, M. & Lust, E.|title= Electrical double-layer characteristics of novel carbide-derived carbon materials|journal=Carbon|volume= 44|pages= 2122–2129 |year=2006|doi=10.1016/j.carbon.2006.04.022|issue=11}}</ref> |
||
| ⚫ | |||
<ref name="a33">Leis, J., Arulepp, M., Kuura, A., Latt, M. & Lust, E. Electrical double-layer characteristics of novel carbide-derived carbon materials. ''Carbon'' 44, 2122–2129 (2006).</ref> |
|||
| ⚫ | |||
| ⚫ | |||
<ref name="a36">{{cite journal|author=Portet, C., Yushin, G. & Gogotsi, Y. |title=Effect of Carbon Particle Size on Electrochemical Performance of EDLC|journal=Journal of the Electrochemical Society|volume= 155|pages= A531–A536 |year=2008|doi=10.1149/1.2918304|issue=7}}</ref> |
|||
| ⚫ | |||
<ref name=" |
<ref name="a37">{{cite journal|author=Presser, V., McDonough, J., Yeon, S.-H. & Gogotsi, Y. |title=Effect of pore size on carbon dioxide sorption by carbide derived carbon|journal=Energy & Environmental Science|volume= 4|pages= 3059–3066 |year=2011|doi=10.1039/c1ee01176f|issue=8}}</ref> |
||
<ref name=" |
<ref name="a38">{{cite journal|author=Vakifahmetoglu, C., Presser, V., Yeon, S.-H., Colombo, P. & Gogotsi, Y. |title=Enhanced hydrogen and methane gas storage of silicon oxycarbide derived carbon|journal=Microporous and Mesoporous Materials|volume= 144|pages= 105–112 |year=2011|doi=10.1016/j.micromeso.2011.03.042}}</ref> |
||
<ref name="a39">{{cite journal|author=Erdemir, A. et al.|title= Effects of High-Temperature Hydrogenation Treatment on Sliding Friction and Wear Behavior of Carbide-Derived Carbon Films|journal=Surface and Coatings Technology|volume= 188|pages= 588–593 |year=2004|doi=10.1016/j.surfcoat.2004.07.052}}</ref> |
|||
<ref name="a38">Vakifahmetoglu, C., Presser, V., Yeon, S.-H., Colombo, P. & Gogotsi, Y. Enhanced hydrogen and methane gas storage of silicon oxycarbide derived carbon. ''Microporous and Mesoporous Materials'' 144, 105–112 (2011).</ref> |
|||
<ref name=" |
<ref name="a40">{{cite journal|author=Carroll, B., Gogotsi, Y., Kovalchenko, A., Erdemir, A. & McNallan, M. J.|title= Effect of Humidity on the Tribological Properties of Carbide-Derived Carbon (CDC) Films on Silicon Carbide|journal=Tribology Letters|volume= 15|pages= 51–55 |year=2003|doi=10.1023/A:1023508006745}}</ref> |
||
<ref name="a41">{{cite journal|author=Yushin, G. et al.|title= Mesoporous carbide-derived carbon with porosity tuned for efficient adsorption of cytokines|journal=Biomaterials|volume= 27 |year=2006|doi=10.1016/j.biomaterials.2006.07.019|issue=34|pages=5755–62|pmid=16914195}}</ref> |
|||
<ref name="a40">Carroll, B., Gogotsi, Y., Kovalchenko, A., Erdemir, A. & McNallan, M. J. Effect of Humidity on the Tribological Properties of Carbide-Derived Carbon (CDC) Films on Silicon Carbide. ''Tribology Letters'' 15, 51-55 (2003).</ref> |
|||
<ref name=" |
<ref name="a42">{{cite journal|author=Yachamaneni, S. et al.|title= Mesoporous carbide-derived carbon for cytokine removal from blood plasma|journal=Biomaterials|volume= 31|pages= 4789–4795 |year=2010|doi=10.1016/j.biomaterials.2010.02.054|issue=18}}</ref> |
||
<ref name="a43">{{cite journal|author=Ersoy, D. A., McNallan, M. J. & Gogotsi, Y.|title= Platinum Reactions with Carbon Coatings Produced by High Temperature Chlorination of Silicon Carbide|journal=Journal of the Electrochemical Society|volume= 148|pages= C774–C779 |year=2001|doi=10.1149/1.1415033|issue=12}}</ref> |
|||
<ref name="a42">Yachamaneni, S. et al. Mesoporous carbide-derived carbon for cytokine removal from blood plasma. ''Biomaterials'' 31, 4789-4795 (2010).</ref> |
|||
<ref name=" |
<ref name="a44">{{cite journal|author=Niu, J. J., Presser, V., Karwacki, C. & Gogotsi, Y. |title=Ultrasmall Gold Nanoparticles with the Size Controlled by the Pores of Carbide-Derived Carbon|journal=Materials Express|volume= 1|pages= 259–266 |year=2011|doi=10.1166/mex.2011.1040|issue=4}}</ref> |
||
| ⚫ | |||
<ref name="a44">Niu, J. J., Presser, V., Karwacki, C. & Gogotsi, Y. Ultrasmall Gold Nanoparticles with the Size Controlled by the Pores of Carbide-Derived Carbon. ''Materials Express'' 1, 259-266 (2011).</ref> |
|||
| ⚫ | |||
}} |
|||
== External links == |
== External links == |
||
| Line 140: | Line 138: | ||
* http://y-carbon.us |
* http://y-carbon.us |
||
* http://skeletontech.com/ |
* http://skeletontech.com/ |
||
{{DISPLAYTITLE:Carbide-Derived Carbon}} |
|||
{{PAGENAME:Carbide-Derived Carbon}} |
|||
{{DEFAULTSORT:Carbide-Derived Carbon}} |
|||
[[Category:Carbon forms]] |
[[Category:Carbon forms]] |
||
[[Category:Capacitors]] |
[[Category:Capacitors]] |
||
Revision as of 13:23, 10 January 2013
Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2).[1][2][3][4] CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C [5][6][7]. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp2- to sp3-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: micro- and mesoporous carbon, amorphous carbon, carbon nanotubes, onion-like carbon, nanocrystalline diamond, graphene, and graphite[1]. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m2/g)[8]. By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy[9]. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO2) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization.
History
The process of using high-temperature chlorine treatment for production of SiCl4 by etching of metal carbides was first patented in 1918 by Otis Hutchins[10], with the process further optimized for higher yields in 1956[11]. The solid porous carbon product was initially regarded as a waste byproduct until its properties and potential applications were investigated in more detail in 1959 by Walter Mohun[12]. Research was carried out in the 1960-1980s mostly by Russian scientists on the synthesis of CDC via halogen treatment[13][14], while hydrothermal treatment was explored as an alternative route to derive CDCs in the 1990s[15]. Most recently, research activities have centered on optimized CDC synthesis and nanoengineered CDC precursors.
Nomenclature
Historically, various terms have been used for CDC, such as “mineral carbon” or “nanoporous carbon”[12]. Later, a more adequate nomenclature was adopted that clearly denotes the precursor. For example, CDC derived from silicon carbide has been referred to as SiC-CDC, Si-CDC, or SiCDC. Recently, it was recommended to adhere to a unified precursor-CDC-nomenclature to reflect the chemical composition of the precursor (e.g., B4C-CDC, Ti3SiC2-CDC, W2C-CDC)[1].
Synthesis
CDCs have been synthesized using several chemical and physical synthesis methods. Most commonly, dry chlorine treatment is used to selectively etch metal or metalloid atoms from the carbide precursor lattice[1]. The term “chlorine treatment” is to be preferred over chlorination as the chlorinated product, metal chloride, is the discarded byproduct and the carbon itself remains largely unreacted. This method is implemented for commercial production of CDC by Skeleton in Estonia and Y-Carbon in the USA. The vacuum decomposition of SiC wafers (i.e., single crystals) is used for the synthesis of epitaxial graphene with homogeneous areas in the cm2 range[16]. Hydrothermal etching has also been used for synthesis of SiC-CDC which yielded a route for porous carbon films and nanodiamond synthesis[17][18].

Chlorine treatment
The most common method for producing porous carbide-derived carbons involves high-temperature etching with halogens, most commonly chlorine gas. The following generic equation describes the reaction of a metal chloride with chlorine gas (M: Si, Ti, V; similar equations can be written for other CDC precursors):
- MC(solid) + 2 Cl2(gas) → MCl4(gas) + C(solid)
Halogen treatment at temperatures between 200 and 1000 °C has been shown to yield mostly disordered porous carbons with a porosity between 50 and ~80 vol% depending on the precursor. Temperatures above 1000 °C result in predominantly graphitic carbon and an observed shrinkage of the material due to graphitization.

The linear growth rate of the solid carbon product phase suggests a reaction-driven kinetic mechanism, but the kinetics become diffusion-limited for thicker films or larger particles. A high mass transport condition (high gas flow rates) facilitates the removal of the chloride and shifts the reaction equilibrium towards the CDC product. Chlorine treatment has successfully been employed for CDC synthesis from a variety of carbide precursors, including SiC, TiC, B4C, BaC2, CaC2, Cr3C2, Fe3C, Mo2C, Al4C3, Nb2C, SrC2, Ta2C, VC, WC, W2C, ZrC, ternary carbides such as Ti2AlC, Ti3AlC2, and Ti3SiC2, and carbonitrides such as Ti2AlC0.5N0.5.
Most produced CDCs exhibit a prevalence of micropores (< 2 nm) and mesopores (between 2 and 50 nm), with specific distributions affected by carbide precursor and synthesis conditions[19]. Hierarchic porosity can be achieved by using polymer-derived ceramics with or without utilizing a templating method[20]. Templating yields an ordered array of mesopores in addition to the disordered network of micropores. It has been shown that the initial crystal structure of the carbide is the primary factor affecting the CDC porosity, especially for low-temperature chlorine treatment. In general, a larger spacing between carbon atoms in the lattice correlates with an increase in the average pore diameter[2][21]. As the synthesis temperature increases, the average pore diameter increases, while the pore size distribution becomes broader[9]. The overall shape and size of the carbide precursor, however, is largely maintained and CDC formation is usually referred to as a conformal process[19].

Vacuum decomposition
Main article: Epitaxial graphene
Metal or metalloid atoms from carbides can selectively be extracted at high temperatures (usually above 1200 °C) under vacuum. The underlying mechanism is incongruent decomposition of carbides, using the high melting point of carbon compared to corresponding carbide metals that melt and eventually evaporate away, leaving the carbon behind[22].
Like halogen treatment, vacuum decomposition is a conformal process[19]. The resulting carbon structures are, as a result of the higher temperatures, more ordered, and carbon nanotubes and graphene can be obtained. In particular, vertically aligned carbon nanotubes films of high tube density have been reported for vacuum decomposition of SiC[23]. The high tube density translates into a high elastic modulus and high buckling resistance which is of particular interest for mechanical and tribological applications[24].
While carbon nanotube formation occurs when trace oxygen amounts are present, very high vacuum conditions (approaching 10−8-10−10 torr) result in the formation of graphene sheets. If the conditions are maintained, graphene transitions into bulk graphite. In particular, by vacuum annealing silicon carbide single crystals (wafers) at 1200–1500 °C[16], metal/metalloid atoms are selectively removed and a layer of 1–3 layer graphene (depending on the treatment time) is formed, undergoing a conformal transformation of 3 layers of silicon carbide into one monolayer of graphene [25]. Also, graphene formation occurs prefentially on the Si-face of the 6H-SiC crystals, while nanotube growth is favored on the c-face of SiC[23].
Hydrothermal decomposition
The removal of metal atoms from carbides has been reported at high temperatures (300–1000 °C) and pressures (2-200 MPa). The following reactions are possible between metal carbides and water:
(a) x/2•MC + x•H2O → Mx/2Ox + x/2•CH4
(b) MC + (x+1)•H2O → MOx + CO + (x+1)•H2
(c) MC + (x+2)•H2O → MOx + CO2 + (x+2)•H2
(d) MC + x•H2O → MOx + C + x•H2
Only reaction (d) yields solid carbon. The favorability of the formation of carbon-containing gases increases with higher pressure (decreasing solid carbon yield) and decreases at higher temperatures (increasing the carbon yield). The ability to produce a usable porous carbon material is dependent on the solubility of the formed metal oxide (such as SiO2) in supercritical water. Hydrothermal carbon formation has been reported for SiC, TiC, WC, TaC, and NbC. Insolubility of metal oxides, for example TiO2, is a significant complication for certain metal carbides (e.g., Ti3SiC2).[19][26]
Applications
Energy storage
One application of carbide-derived carbons is as active material in electrodes for electric double layer capacitors which have become commonly known as supercapacitors or ultracapacitors. This is motivated by their good electrical conductivity combined with high surface area[27], large micropore volume[21], and pore size control[28] that enable to match the porosity metrics of the porous carbon electrode to a certain electrolyte[29]. In particular, when the pore size approaches the size of the (desolvated) ion in the electrolyte, there is a significant increase in the capacitance. The electrically conductive carbon material minimizes resistance losses in supercapacitor devices and enhances charge screening and confinement[30], maximizing the packing density and subsequent charge storage capacity of microporous CDC electrodes[31][32][33].
CDC electrodes have been shown to yield a gravimetric capacitance of up to 190 F/g in aqueous electrolytes and 180 F/g in organic electrolytes[29]. The highest capacitance values are observed for matching ion/pore systems, which allow high-density packing of ions in pores in superionic states[34]. However, small pores, especially when combined with an overall large particle diameter, impose an additional diffusion limitation on the ion mobility during charge/discharge cycling. The prevalence of mesopores in the CDC structure allows for more ions to move past each other during charging and discharging, allowing for faster scan rates and improved rate handling abilities[35]. Conversely, by implementing nanoparticle carbide precursors, shorter pore channels allow for higher electrolyte mobility, resulting in faster charge/discharge rates and higher power densities [36].
Gas storage and carbon dioxide capturing
TiC-CDC activated with KOH or CO2 has been shown to store up to 21 wt.% of methane at 25 °C at high pressure. In particular, CDCs with subnanometer pores in the 0.50–0.88 nm diameter range have shown to store up to 7.1 mol CO2/kg at 1 bar and 0 °C.[37] CDCs have also been shown to store up to 3 wt.% hydrogen at 60 bar and −196 °C, with additional increases possible as a result of chemical or physical activation of the CDC materials. SiOC-CDC with large subnanometer pore volumes are able to store over 5.5 wt.% hydrogen at 60 bar and −196 °C, almost reaching the goal of the US Department of Energy of 6 wt.% storage density for automotive applications. Methane storage densities of over 21.5 wt.% can be achieved for this material at those conditions. In particular, a predominance of pores with subnanometer diameters and large pore volumes are instrumental towards increasing storage densities[38].
Tribological coatings
CDC films obtained by vacuum annealing (ESK) or chlorine treatment of SiC ceramics yield a low friction coefficient. The friction coefficient of SiC, which is widely used in tribological applications for its high mechanical strength and hardness, can therefore decrease from ~0.7 to ~0.2 or less under dry conditions[39]. It’s important to mention that graphite cannot operate in dry environments. The porous 3-dimensional network of CDC allows for high ductility and an increased mechanical strength, minimizing fracture of the film under an applied force. Those coatings find applications in dynamic seals. The friction properties can be further tailored with high-temperature hydrogen annealing and subsequent hydrogen termination of dangling bonds [40].
Protein adsorption
Carbide-derived carbons with a mesoporous structure have been shown to remove large molecules from biofluids. As other carbons, CDCs possess good biocompatibility[41]. CDCs have been demonstrated to remove cytokines such as TNF-alpha, IL-6, and IL-1beta from blood plasma. These are the most common receptor-binding agents released into the body during a bacterial infection that cause the primary inflammatory response during the attack and increase the potential lethality of sepsis, making their removal a very important concern[42]. The rates and levels of removal of above cytokines (85–100% removed within 30 minutes) are higher than those observed for comparable activated carbons[42].
Catalyst support
It has been demonstrated that Pt nanoparticles can be introduced to the SiC/C interface during chlorine treatment (in the form of Pt3Cl3). The particles diffuse through the material to form Pt particle surfaces, which may serve as catalyst support layers[43]. In particular, in addition to Pt, other noble elements such as gold can be deposited into the pores, with the resulting nanoparticle size controlled by the pore size and overall pore size distribution of the CDC substrate[44]. Such gold or platinum nanoparticles can be smaller than 1 nm even without employing surface coatings[44]. Au nanoparticles in different CDCs (TiC-CDC, Mo2C-CDC, B4C-CDC) have been successfully used as catalyst for carbon monoxide oxidation without the presence of a traditional oxide support[44].
Capacitive deionization (CDI)
As desalinization and purification of water is critical for obtaining deionized water for laboratory research, large-scale chemical synthesis in industry and consumer applications, the use of porous materials for this application has received particular interest. Capacitive deionization operates in a fashion with similarities to a supercapacitor. As an ion-containing water (electrolyte) is flown between two porous electrodes with an applied potential across the system, the corresponding ions assemble into a double layer in the pores of the two terminals, decreasing the ion content in the liquid exiting the purification device[45]. Due to the ability of carbide-derived carbons to closely match the size of ions in the electrolyte, side-by-side comparisons of desalinization devices based on CDCs and activated carbon showed a significant efficiency increase in the 1.2–1.4 V range compared to activated carbon[45].
Commercial production and applications
Having originated as the by-product of industrial metal chloride synthesis, CDC has certainly a potential for large-scale production at a moderate cost. Currently, two small companies engage in scalable production of carbide-derived carbons and their implementation in commercial products. Skeleton, which is located in Tartu, Estonia, has a diverse product line of porous carbons for supercapacitors, gas storage, and filtration applications. Y-Carbon, which is based in Bristol, Pennsylvania (United States), is also involved in the production and development of carbide-derived carbons for water desalination, special sorbents, chromatography, filtration, and electrical energy storage and generation. In addition, numerous education and research institutions worldwide are engaged in basic research of CDC structure, synthesis, or (indirectly) their application for various high-end applications.
See also
References
- ^ a b c d Presser, V., Heon, M. & Gogotsi, Y. (2011). "Carbide-Derived Carbons – From Porous Networks to Nanotubes and Graphene". Advanced Functional Materials. 21 (5): 810–833. doi:10.1002/adfm.201002094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kyotani, T., Chmiola, J. & Gogotsi, Y. in Carbon Materials for Electrochemical Energy Storage Systems (eds F. Beguin & E. Frackowiak) Ch. 3, 77–113 (CRC Press/Taylor and Francis, 2009).
- ^ Yushin, G., Nikitin, A. & Gogotsi, Y. (2006) in Carbon Nanomaterials, Y. Gogotsi (ed.) pp. 211–254, CRC Taylor & Francis ISBN 0849393868.
- ^ Nikitin, A. & Gogotsi, Y. (2004) in Encyclopedia of Nanoscience and Nanotechnology Vol. 7, H.S. Nalwa (ed.) pp. 553–574, American Scientific Publishers
- ^ Rose, M.; et al. (2011). "Hierarchical Micro- and Mesoporous Carbide-Derived Carbon as a High-Performance Electrode Material in Supercapacitors". Small. 7 (8): 1108–1117. doi:10.1002/smll.201001898. PMID 21449047.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Yeon, S.-H.; et al. (2010). "Carbide-derived-carbons with hierarchical porosity from a preceramic polymer". Carbon. 48: 201–210. doi:10.1016/j.carbon.2009.09.004.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Presser, V.; et al. (2011). "Flexible Nano-Felts of Carbide-Derived Carbon with Ultra-High Power Handling Capability". Advanced Energy Materials. 1 (3): 423–430. doi:10.1002/aenm.201100047.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Rose, M., Kockrick, E., Senkovska, I. & Kaskel, S. (2010). "High surface area carbide-derived carbon fibers produced by electrospinning of polycarbosilane precursors". Carbon. 48 (2): 403–407. doi:10.1016/j.carbon.2009.09.043.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gogotsi, Y.; et al. (2003). "Nanoporous carbide-derived carbon with tunable pore size". Nature Materials. 2 (9): 591–594. doi:10.1038/nmat957. PMID 12907942.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Hutchins, O. Method for the Production of Silicon Tetrachlorid. U.S. patent 1,271,713 (1918)
- ^ Andersen, J. N. Silicon Tetrachloride Manufacture. U.S. patent 2,739,041 (1956)
- ^ a b Mohun, W. A. in Proceedings of the Conference on Carbon Vol. 4 pp. 443–453 (1959)
- ^ Babkin, O. E., Ivakhnyuk, G. K., Lukin, Y. N. & Fedorov, N. F. (1988). "Study of structure of carbide derived carbon by XPS". Zhurnal Prikladnoi Khimii. 57: 1719–1721.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gordeev, S. K. & Vartanova, A. V. (1994). "New approach for production of block microporous materials". Zhurnal Prikladnoi Khimii. 67: 1375–1377.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yoshimura, M. et al. Dense Carbon Coating on Silicon Carbide Finers by Hydrothermal Treatment. International Symposium on Carbon, Tokyo, Japan; The Carbon Society of Japan, 552–553 (1998).
- ^ a b Lee, D. S.; et al. (2008). "Raman Spectra of Epitaxial Graphene on SiC and of Epitaxial Graphene Transferred to SiO2". Nano Letters. 8 (12): 4320–4325. doi:10.1021/nl802156w. PMID 19368003.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Roy, R., Ravichandran, D., Badzian, A. & Breval, E. (1996). "Attempted hydrothermal synthesis of diamond by hydrolysis of b-SiC powder". Diamond and Related Materials. 5 (9): 973–976. doi:10.1016/0925-9635(95)00443-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kitaoka, S., Tsuji, T., Katoh, T. & Yamaguchi, Y. (1994). "Tribological Characteristics of SiC Ceramics in High-Temperature and High-Pressure Water". Journal of the American Ceramic Society. 77 (7): 1851–1856. doi:10.1111/j.1151-2916.1994.tb07061.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Presser, V., Heon, M. & Gogotsi, Y. (2011). "Carbide-Derived Carbons-From Porous Networks to Nanotubes and Graphene". Advanced Functional Materials. 21 (5): 810–833. doi:10.1002/adfm.201002094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kockrick, E.; et al. (2010). "Ordered mesoporous carbide derived carbons for high pressure gas storage". Carbon. 48 (6): 1707–1717. doi:10.1016/j.carbon.2010.01.004.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Arulepp, M.; et al. (2006). "The advanced carbide-derived carbon based supercapacitor". Journal of Power Sources. 162 (2): 1460–1466. doi:10.1016/j.jpowsour.2006.08.014.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Kosolapova, T. Y. Carbides. Properties, Production, and Applications. (Plenum Press, 1971.
{{cite journal}}: Italic or bold markup not allowed in:|journal=(help); Missing or empty|title=(help) - ^ a b Kusunoki, M., Rokkaku, M. & Suzuki, T. (1997). "Epitaxial carbon nanotube film self-organized by sublimation decomposition of silicon carbide". 71: 2620–2622.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Pathak, S., Cambaz, Z. G., Kalidindi, S. R., Swadener, J. G. & Gogotsi, Y. (2009). "Viscoelasticity and high buckling stress of dense carbon nanotube brushes". Carbon. 47 (8): 1969–1976. doi:10.1016/j.carbon.2009.03.042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zhou, H.; et al. (2012). "Understanding controls on interfacial wetting at epitaxial graphene: Experiment and theory". Physical Review B. 85 (3): 035406. doi:10.1103/PhysRevB.85.035406.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Hoffman, E. N., Yushin, G., El-Raghy, T., Gogotsi, Y. & Barsoum, M. W. (2008). "Micro and mesoporosity of carbon derived from ternary and binary metal carbides". Microporous and Mesoporous Materials. 112: 526–532. doi:10.1016/j.micromeso.2007.10.033.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pandolfo, A. G. & Hollenkamp, A. F. (2006). "Carbon properties and their role in supercapacitors". Journal of Power Sources. 157: 11–27. doi:10.1016/j.jpowsour.2006.02.065.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Simon, P. & Gogotsi, Y. (2008). "Materials for electrochemical capacitors". Nature Materials. 7 (11): 845–854. doi:10.1038/nmat2297. PMID 18956000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Chmiola, J.; et al. (2006). "Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer". Science. 313 (5794): 1760–1763. doi:10.1126/science.1132195. PMID 16917025.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Huang, J.; et al. (2010). "Curvature effects in carbon nanomaterials: Exohedral versus endohedral supercapacitors". Journal of Materials Research Society. 25 (8): 1525–1531. doi:10.1557/JMR.2010.0195.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Huczko, A.; et al. (2007). "Characterization of 1-D nanoSiC-derived nanoporous carbon". Physica Status Solidi B. 244 (11): 3969–3972. doi:10.1002/pssb.200776162.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Permann, L., Latt, M., Leis, J. & Arulepp, M. (2006). "Electrical double layer characteristics of nanoporous carbon derived from titanium carbide". Electrochimica Acta. 51 (7): 1274–1281. doi:10.1016/j.electacta.2005.06.024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leis, J., Arulepp, M., Kuura, A., Latt, M. & Lust, E. (2006). "Electrical double-layer characteristics of novel carbide-derived carbon materials". Carbon. 44 (11): 2122–2129. doi:10.1016/j.carbon.2006.04.022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kondrat, S. & Kornyshev, A. (2011). "Superionic state in double-layer capacitors with nanoporous electrodes". Journal of Physics: Condensed Matter. 23 (2): 022201. doi:10.1088/0953-8984/23/2/022201.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fulvio, P. F.; et al. (2011). ""Brick-and-Mortar" Self-Assembly Approach to Graphitic Mesoporous Carbon Nanocomposites". Advanced Functional Materials. 21 (12): 2208–2215. doi:10.1002/adfm.201002641.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Portet, C., Yushin, G. & Gogotsi, Y. (2008). "Effect of Carbon Particle Size on Electrochemical Performance of EDLC". Journal of the Electrochemical Society. 155 (7): A531–A536. doi:10.1149/1.2918304.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Presser, V., McDonough, J., Yeon, S.-H. & Gogotsi, Y. (2011). "Effect of pore size on carbon dioxide sorption by carbide derived carbon". Energy & Environmental Science. 4 (8): 3059–3066. doi:10.1039/c1ee01176f.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vakifahmetoglu, C., Presser, V., Yeon, S.-H., Colombo, P. & Gogotsi, Y. (2011). "Enhanced hydrogen and methane gas storage of silicon oxycarbide derived carbon". Microporous and Mesoporous Materials. 144: 105–112. doi:10.1016/j.micromeso.2011.03.042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Erdemir, A.; et al. (2004). "Effects of High-Temperature Hydrogenation Treatment on Sliding Friction and Wear Behavior of Carbide-Derived Carbon Films". Surface and Coatings Technology. 188: 588–593. doi:10.1016/j.surfcoat.2004.07.052.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Carroll, B., Gogotsi, Y., Kovalchenko, A., Erdemir, A. & McNallan, M. J. (2003). "Effect of Humidity on the Tribological Properties of Carbide-Derived Carbon (CDC) Films on Silicon Carbide". Tribology Letters. 15: 51–55. doi:10.1023/A:1023508006745.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yushin, G.; et al. (2006). "Mesoporous carbide-derived carbon with porosity tuned for efficient adsorption of cytokines". Biomaterials. 27 (34): 5755–62. doi:10.1016/j.biomaterials.2006.07.019. PMID 16914195.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Yachamaneni, S.; et al. (2010). "Mesoporous carbide-derived carbon for cytokine removal from blood plasma". Biomaterials. 31 (18): 4789–4795. doi:10.1016/j.biomaterials.2010.02.054.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Ersoy, D. A., McNallan, M. J. & Gogotsi, Y. (2001). "Platinum Reactions with Carbon Coatings Produced by High Temperature Chlorination of Silicon Carbide". Journal of the Electrochemical Society. 148 (12): C774–C779. doi:10.1149/1.1415033.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Niu, J. J., Presser, V., Karwacki, C. & Gogotsi, Y. (2011). "Ultrasmall Gold Nanoparticles with the Size Controlled by the Pores of Carbide-Derived Carbon". Materials Express. 1 (4): 259–266. doi:10.1166/mex.2011.1040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Porada, S.; et al. (2012). "Water Desalination Using Capacitive Deionization with Microporous Carbon Electrodes". ACS Applied Materials & Interfaces. 4 (3): 1194–1199. doi:10.1021/am201683j.
{{cite journal}}: Explicit use of et al. in:|author=(help)
