Blastulation: Difference between revisions
m →Structure: ~~~~ |
~~~~ added development section |
||
| Line 26: | Line 26: | ||
The study of the blastula and of cell specification has many implications on the field of [[embryonic stem cells|stem cell]] research as well as the continued improvement of fertility treatments.<ref name=Cockburn /> Embryonic stem cells are a field which, though controversial, have tremendous potential for treating disease. In Xenopus, blastula behave as pluripotent stem cells which can migrate down several pathways, depending on signaling. <ref name=Gurdon /> By manipulating the signaling factors, various tissues can be formed. This potential can be instrumental in [regenerative medicine] for disease and injury cases. [[In vitro fertilisation]] involves implantation of a blastula into a mother’s uterus.<ref name=Toth /> Blastula cell implantation could potentially serve to eliminate infertility. |
The study of the blastula and of cell specification has many implications on the field of [[embryonic stem cells|stem cell]] research as well as the continued improvement of fertility treatments.<ref name=Cockburn /> Embryonic stem cells are a field which, though controversial, have tremendous potential for treating disease. In Xenopus, blastula behave as pluripotent stem cells which can migrate down several pathways, depending on signaling. <ref name=Gurdon /> By manipulating the signaling factors, various tissues can be formed. This potential can be instrumental in [regenerative medicine] for disease and injury cases. [[In vitro fertilisation]] involves implantation of a blastula into a mother’s uterus.<ref name=Toth /> Blastula cell implantation could potentially serve to eliminate infertility. |
||
==Development== |
|||
The blastocoel, the defining feature of the blastula, forms as a product of the repeated cleavage of the cells into the blastomeres and the secretion of fluid. The development of the early blastula from the zygote through the cleavage process is controlled by maternal mRNA produced in oogenesis before fertilisation.<ref>{{cite journal|last=Tadros|first=W|coauthors=Lipshitz, HD|title=Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila.|journal=Developmental dynamics : an official publication of the American Association of Anatomists|date=2005 Mar|volume=232|issue=3|pages=593-608|pmid=15704150}}</ref> This is possibly due to the fact that early nuclear division during cleavage is too rapid to allow the molecular machinery of transcription to function.<ref>{{cite journal|last=Etkin|first=LD|title=Regulation of the mid-blastula transition in amphibians.|journal=Developmental biology (New York, N.Y. : 1985)|date=1988|volume=5|pages=209-25|pmid=3077975}}</ref> |
|||
===Mid-blastula Transition=== |
|||
The mid-blastula transition is defined by the ending of the synchronous cell division cycles of the early blastula development, lengthening of the cell cycles by the addition of the G1 and G2 phases.<ref>{{cite journal|last=Etkin|first=LD|title=Regulation of the mid-blastula transition in amphibians.|journal=Developmental biology (New York, N.Y. : 1985)|date=1988|volume=5|pages=209-25|pmid=3077975}}</ref> The mid-blastula transition is also characterised by a marked increase in transcription of new, non-maternal mRNA. Large amounts of the maternal mRNA are destroyed at this point, either by proteins such as SMAUG in drosophilae<ref>{{cite journal|last=Tadros|first=W|coauthors=Westwood, JT; Lipshitz, HD|title=The mother-to-child transition.|journal=Developmental cell|date=2007 Jun|volume=12|issue=6|pages=847-9|pmid=17543857}}</ref> or microRNA.<ref>{{cite journal|last=Weigel|first=D|coauthors=Izaurralde, E|title=A tiny helper lightens the maternal load.|journal=Cell|date=2006 Mar 24|volume=124|issue=6|pages=1117-8|pmid=16564001}}</ref> |
|||
==Structure== |
==Structure== |
||
Revision as of 15:59, 4 April 2013
| Blastula | |
|---|---|
 Blastulation: 1 - morula, 2 - blastula. | |
| Details | |
| Days | 4 |
| Precursor | Morula |
| Gives rise to | Gastrula |
| Anatomical terminology | |
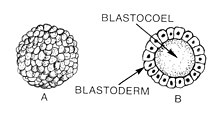
The blastula (from Greek βλαστός (blastos), meaning "sprout") is a hollow sphere of cells, referred to as blastomeres, surrounding an inner fluid-filled cavity called the blastocoele formed during an early stage of embryonic development in animals.[1]Embryo development begins with a sperm fertilizing an egg to become a zygote which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form. [2]
A common feature of a vertebrate blastula is that it consists of a layer of blastomeres, known as the blastoderm, which surrounds the blastocoel.[3] [4] In mammals, blastulation leads to the formation of the blastocyst, instead of the blastula. The blastocyst contains an embryoblast (or inner cell mass) that will eventually give rise to the definitive structures of the fetus, and the trophoblast, which goes on to form the extraembryonic tissues.[citation needed][2]
During the blastula stage of developoment, a significant amount of activity occurs within the early embryo to establish cell polarity, axis formation, and regulate gene expression.[2] In amphibians, the mid blastula transition (MBT) is a crucial step in development during which the maternal mRNA is degraded and control over development is passed to the embryo.[5] Many of the interactions between blastomeres are dependent on cadherin expression, particularly E-cadherin in mammals and EP-cadherin in amphibians.[6]
The study of the blastula and of cell specification has many implications on the field of stem cell research as well as the continued improvement of fertility treatments.[7] Embryonic stem cells are a field which, though controversial, have tremendous potential for treating disease. In Xenopus, blastula behave as pluripotent stem cells which can migrate down several pathways, depending on signaling. [8] By manipulating the signaling factors, various tissues can be formed. This potential can be instrumental in [regenerative medicine] for disease and injury cases. In vitro fertilisation involves implantation of a blastula into a mother’s uterus.[9] Blastula cell implantation could potentially serve to eliminate infertility.
Development
The blastocoel, the defining feature of the blastula, forms as a product of the repeated cleavage of the cells into the blastomeres and the secretion of fluid. The development of the early blastula from the zygote through the cleavage process is controlled by maternal mRNA produced in oogenesis before fertilisation.[10] This is possibly due to the fact that early nuclear division during cleavage is too rapid to allow the molecular machinery of transcription to function.[11]
Mid-blastula Transition
The mid-blastula transition is defined by the ending of the synchronous cell division cycles of the early blastula development, lengthening of the cell cycles by the addition of the G1 and G2 phases.[12] The mid-blastula transition is also characterised by a marked increase in transcription of new, non-maternal mRNA. Large amounts of the maternal mRNA are destroyed at this point, either by proteins such as SMAUG in drosophilae[13] or microRNA.[14]
Structure
Generally speaking, a blastula is a sphere of cells surrounding a blastocoel. The blastocoel is a fluid filled cavity which contains amino acids, proteins, growth factors, sugars ions and other components which are necessary for cellular differentiation while also allowing blastomeres to move during the process of gastrulation.[15]
In Xenopus embryos, the blastula is composed of three different regions. The animal cap forms the roof of the blastocoel and goes on primarily to form ectodermal derivatives. The equatorial or marginal zone, which compose the walls of the blastocoel differentiate primarily into mesodermal tissue. The vegetal mass is composed of the blastocoel floor and primarily develops into endodermal tissue.[6]
In the mammalian blastocyst (term for mammalian blastula) there are three lineages which give rise to later tissue development. The epiblast gives rise to the fetus itself while the trophectoderm develops into part of the placenta and the primitive endoderm becomes the yolk sac.[7]
In mouse embryo, blastocoel formation begins at the 32-cell stage. During this process, water enters the embryo, aided by an osmotic gradient which is the result of Na+/K+ ATPases that produce a high Na+ gradient on the basolateral side of the trophectoderm. This movement of water is facilitated by aquaporins. A seal is created by tight junctions of the epithelial cells which line the blastocoel.[7] Cell-cell interactions
Cellular adhesion
Tight junctions are very important in embryo development. In the blastula, these cadherin mediated cell interactions are essential to development of epithelial layers which are important to paracellular transport, maintenance of cell polarity and the creation of a permeability seal to regulate blastocoel formation. In both Xenopus and mammalian embryos, tight junctions arise after epithelial polarity is established. Within the blastula, inner blastomeres are generally non-polar while epithelial cells demonstrate polarity.[15]
Mammalian embryos undergo compaction around the 8-cell stage where E-cadherins as well as alpha and beta catenins are expressed. E-cadherin adhesion defines the apico-basal axis in the developing embryo and turns the embryo from an indistinct ball of cells to a more polarized phenotype which sets the stage for further development into a fully formed blastocyst. (Fleming)[15]
Xenopus membrane polarity is established with the first cell cleavage. Amphibian EP-cadherin and XB/U cadherin perform a similar role as E-cadherin in mammals establishing blastomere polarity and solidifying cell-cell interactions which are crucial for further development.[15]
Clinical Implications
Fertilization technologies
Experiments with implantation in mice show that hormonal induction, superovulation and artificial insemination successfully produce preimplantion mice embryos. In the mice, ninety percent of the females were induced by mechanical stimulation to undergo pregnancy and implant at least one embryo. [16] These results prove to be encouraging because they provide a basis for potential implantation in other mammalian species, such as humans.
Stem cells
Blastula-stage cells can behave as pluripotent stem cells in many species. Pluripotent stem cells are the starting point to produce organ specific cells that can potentially aid in repair and prevention of injury and degenerations, respectively. Combining the expression of transcription factors and locational positioning of the blastula cells can lead to the development of induced functional organs and tissues. Pluripotent Xenopus laevis cells, when used in an in vivo strategy, were able to form into functional retinas. By transplanting them to the eye field, and by inducing several mis-expressions of transcription factors, the cells were committed to the retinal lineage and could guide vision based behavior in the Xenopus. [17]
See also
Notes
[2] [7] [18] [5] [6] [9] [8] [15] [17] [16]
- ^ http://www.britannica.com/EBchecked/topic/69108/blastula
- ^ a b c d Gilbert, Scott (2010). Developmental Biology 9th Ed + Devbio Labortatory Vade Mecum3. Sinauer Associates Inc. ISBN 978-0-87893-558-1.
- ^ Lombardi, Julian (1998). "Embryogenesis". Comparative vertebrate reproduction. Springer. p. 226. ISBN 978-0-7923-8336-9.
- ^ Forgács & Newman, 2005: p. 27
- ^ a b Tadros, Wael (1). "Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation inDrosophila". Developmental Dynamics. 232 (3): 593–608. doi:10.1002/dvdy.20297.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c Heasman, J (1997 Nov). "Patterning the Xenopus blastula". Development (Cambridge, England). 124 (21): 4179–91. PMID 9334267.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d Cockburn, Katie (1 April 2010). "Making the blastocyst: lessons from the mouse". Journal of Clinical Investigation. 120 (4): 995–1003. doi:10.1172/JCI41229.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Gourdon, John B. "Uncommitted Xenopus blastula cells can be directed to uniform muscle gene expression by gradient interpretation and a community effect". The International Journal of Developmental Biology (Cambridge, UK). 46 (8): 993. PMID 12533022.
{{cite journal}}: Cite has empty unknown parameter:|1=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Toth, Attila. "Treatment: Addressing the Causes of Infertility in Men and Women". Macleod Laboratory. Retrieved 22 March 2013.
- ^ Tadros, W (2005 Mar). "Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila". Developmental dynamics : an official publication of the American Association of Anatomists. 232 (3): 593–608. PMID 15704150.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Etkin, LD (1988). "Regulation of the mid-blastula transition in amphibians". Developmental biology (New York, N.Y. : 1985). 5: 209–25. PMID 3077975.
- ^ Etkin, LD (1988). "Regulation of the mid-blastula transition in amphibians". Developmental biology (New York, N.Y. : 1985). 5: 209–25. PMID 3077975.
- ^ Tadros, W (2007 Jun). "The mother-to-child transition". Developmental cell. 12 (6): 847–9. PMID 17543857.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Weigel, D (2006 Mar 24). "A tiny helper lightens the maternal load". Cell. 124 (6): 1117–8. PMID 16564001.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e Fleming, Tom P. (1). "Assembly of tight junctions during early vertebrate development". Seminars in Cell & Developmental Biology. 11 (4): 291–299. doi:10.1006/scdb.2000.0179.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Watson, J.G. "Collection and Transfer of Preimplantation Mouse Embryos". Biology of Reproduction. 17 (3). PMID 901897. Retrieved 01 April 2013.
{{cite journal}}: Check date values in:|accessdate=(help) - ^ a b Viczian, Andrea S. "Generation of Functional Eyes from Pluripotent Cells". PloS Biology. 7 (8). PMID 19688031. Retrieved 01 April 2013.
{{cite journal}}: Check date values in:|accessdate=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Takaoka, K. (6). "Cell fate decisions and axis determination in the early mouse embryo". Development. 139 (1): 3–14. doi:10.1242/dev.060095.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
Bibliography
- "Cleavage and blastula formation". Biological physics of the developing embryo. Cambridge University Press. 2005. ISBN 978-0-521-78337-8.
{{cite book}}: Cite uses deprecated parameter|authors=(help)
Further reading
- Cullen, K.E. (2009). "embryology and early animal development". Encyclopedia of life science, Volume 2. Infobase. ISBN 978-0-8160-7008-4.
- McGeady, Thomas A., ed. (2006). "Gastrulation". Veterinary embryology. Wiley-Blackwell. ISBN 978-1-4051-1147-8.
