Muscle atrophy: Difference between revisions
combined repeated citations |
add citations, expand content on exercise |
||
| Line 21: | Line 21: | ||
| deaths = |
| deaths = |
||
}} |
}} |
||
'''Muscle atrophy''' is the loss of skeletal [[muscle]] mass that can be caused by immobility, aging, malnutrition, medications, or a wide range of injuries or diseases that impact the musculoskeletal or nervous system. Muscle atrophy |
'''Muscle atrophy''' is the loss of skeletal [[muscle]] mass that can be caused by immobility, aging, malnutrition, medications, or a wide range of injuries or diseases that impact the musculoskeletal or nervous system. Muscle atrophy leads to muscle weakness and cause disability. |
||
Disuse causes rapid muscle atrophy and often occurs during injury or illness that requires immobilization of a limb or bed rest. Depending on the duration of disuse, this |
Disuse causes rapid muscle atrophy and often occurs during injury or illness that requires immobilization of a limb or bed rest. Depending on the duration of disuse and the health of the individual, this may be fully reversed with activity. Malnutrition first causes fat loss but may progress to muscle atrophy in prolonged starvation and can be reversed with nutritional therapy. In contrast, [[Cachexia|cachexia]] is a wasting syndrome caused by an underlying disease such as cancer that causes dramatic muscle atrophy and cannot be completely reversed with nutritional therapy. [[Sarcopenia]] is the muscle atrophy associated with aging and can be slowed by exercise. Finally, diseases of the muscles such as [[muscular dystrophy]] or [[myopathies]] can cause atrophy, as well as damage to the nervous system such as in [[spinal cord injury]] or [[stroke]]. |
||
Muscle atrophy results from an imbalance between protein synthesis and protein degradation, although the mechanisms are incompletely understood and are |
Muscle atrophy results from an imbalance between protein synthesis and protein degradation, although the mechanisms are incompletely understood and are variable depending on the cause. Muscle loss can be quantified with advanced imaging studies but this is not frequently pursued. Treatment depends on the underlying cause but will often include exercise and adequate nutrition. [[Anabolic steroid|Anabolic agents]] may have some efficacy but are not often used due to side effects. There are multiple treatments and supplements under investigation but there are currently limited treatment options in clinical practice. Given the implications of muscle atrophy and limited treatment options, minimizing immobility is critical in injury or illness. |
||
==Signs and symptoms== |
==Signs and symptoms== |
||
| Line 71: | Line 71: | ||
==Treatment== |
==Treatment== |
||
Treatment approaches include impacting the signaling pathways that induce [[muscle hypertrophy]] or slow muscle breakdown as well as optimizing nutritional status. |
|||
Exercise is a crucial component to slowing or reversing muscle atrophy. It is still unknown regarding the ideal exercise "dosing." Resistance exercise has been shown to be beneficial in reducing muscle atrophy in older adults.<ref>{{Cite journal|last=Sayer|first=Avan Aihie|date=2014|title=Sarcopenia the new geriatric giant: time to translate research findings into clinical practice|url=https://www.ncbi.nlm.nih.gov/pubmed/25227204|journal=Age and Ageing|volume=43|issue=6|pages=736–737|doi=10.1093/ageing/afu118|issn=1468-2834|pmid=25227204|via=}}</ref><ref>{{Cite journal|last=Liu|first=Chiung-Ju|last2=Latham|first2=Nancy K.|date=2009-07-08|title=Progressive resistance strength training for improving physical function in older adults|url=https://www.ncbi.nlm.nih.gov/pubmed/19588334|journal=The Cochrane Database of Systematic Reviews|issue=3|pages=CD002759|doi=10.1002/14651858.CD002759.pub2|issn=1469-493X|pmc=4324332|pmid=19588334}}</ref> In patients who cannot exercise due to physical limitations, include the include the use of [[functional electrical stimulation]] to stimulate the muscles. This has seen a large amount of success in the rehabilitation of paraplegic patients.<ref>D.Zhang et al., Functional Electrical Stimulation in Rehabilitation Engineering: A survey, Nenyang technological University, Singapore</ref> |
|||
| ⚫ | [[β-Hydroxy β-methylbutyrate]] (HMB), a metabolite of [[leucine]] which is sold as a [[dietary supplement]], has demonstrated efficacy in preventing the loss of muscle mass in several muscle wasting conditions in humans, particularly [[sarcopenia]].<ref name="Sarcopenia July 2015 review">{{cite journal | vauthors = Phillips SM | title = Nutritional supplements in support of resistance exercise to counter age-related sarcopenia | journal = Advances in Nutrition | volume = 6 | issue = 4 | pages = 452–60 | date = July 2015 | pmid = 26178029 | pmc = 4496741 | doi = 10.3945/an.115.008367 }}</ref><ref name="Review April 2016">{{cite journal | vauthors = Brioche T, Pagano AF, Py G, Chopard A | title = Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention | journal = Molecular Aspects of Medicine | volume = 50 | issue = | pages = 56–87 | date = August 2016 | pmid = 27106402 | doi = 10.1016/j.mam.2016.04.006 | quote = In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is cheap (about US$30–50 per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks. (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions, especially in aged people. }}</ref><ref name="Meta-analytic systematic review September 2015">{{cite journal | vauthors = Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K | display-authors = 6 | title = Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis | journal = Archives of Gerontology and Geriatrics | volume = 61 | issue = 2 | pages = 168–75 | date = September 2015 | pmid = 26169182 | doi = 10.1016/j.archger.2015.06.020 | quote = RESULTS: A total of seven randomized controlled trials were included, in which 147 older adults received HMB intervention and 140 were assigned to control groups. The meta-analysis showed greater muscle mass gain in the intervention groups compared with the control groups (standard mean difference=0.352kg; 95% confidence interval: 0.11, 0.594; Z value=2.85; P=0.004). There were no significant fat mass changes between intervention and control groups (standard mean difference=-0.08kg; 95% confidence interval: -0.32, 0.159; Z value=0.66; P=0.511).<br />CONCLUSION: Beta-hydroxy-beta-methylbutyrate supplementation contributed to preservation of muscle mass in older adults. HMB supplementation may be useful in the prevention of muscle atrophy induced by bed rest or other factors. Further studies are needed to determine the precise effects of HMB on muscle strength and physical function in older adults. }}</ref> A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, [[Muscle#Physiology|muscle function]], and [[Muscle#Strength|muscle strength]] in hypercatabolic disease states such as cancer cachexia |
||
| ⚫ | Adequate calories and protein is crucial to prevent muscle atrophy. Supplementation of protein or [[Branched-chain amino acid|branched-chain amino acids]], especially leucine, can provide a stimulus for muscle synthesis and inhibit protein breakdown and has been studied for muscle atrophy for sarcopenia and cachexia.<ref>{{Cite journal|last=Sakamoto|first=N.|last2=Enomoto|first2=N.|last3=Kurosaki|first3=M.|last4=Asahina|first4=Y.|last5=Maekawa|first5=S.|last6=Koizumi|first6=K.|last7=Sakuma|first7=I.|last8=Murakami|first8=T.|last9=Marumo|first9=F.|last10=Sato|first10=C.|date=1995|title=Comparison of the hypervariable region of hepatitis C virus genomes in plasma and liver|url=https://www.ncbi.nlm.nih.gov/pubmed/7623010|journal=Journal of Medical Virology|volume=46|issue=1|pages=7–11|doi=10.1002/jmv.1890460103|issn=0146-6615|pmid=7623010|via=}}</ref><ref>{{Cite journal|last=Argilés|first=Josep M.|last2=Campos|first2=Nefertiti|last3=Lopez-Pedrosa|first3=José M.|last4=Rueda|first4=Ricardo|last5=Rodriguez-Mañas|first5=Leocadio|date=2016|title=Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease|url=https://linkinghub.elsevier.com/retrieve/pii/S152586101630113X|journal=Journal of the American Medical Directors Association|language=en|volume=17|issue=9|pages=789–796|doi=10.1016/j.jamda.2016.04.019|via=}}</ref> [[β-Hydroxy β-methylbutyrate]] (HMB), a metabolite of [[leucine]] which is sold as a [[dietary supplement]], has demonstrated efficacy in preventing the loss of muscle mass in several muscle wasting conditions in humans, particularly [[sarcopenia]].<ref name="Sarcopenia July 2015 review">{{cite journal | vauthors = Phillips SM | title = Nutritional supplements in support of resistance exercise to counter age-related sarcopenia | journal = Advances in Nutrition | volume = 6 | issue = 4 | pages = 452–60 | date = July 2015 | pmid = 26178029 | pmc = 4496741 | doi = 10.3945/an.115.008367 }}</ref><ref name="Review April 2016">{{cite journal | vauthors = Brioche T, Pagano AF, Py G, Chopard A | title = Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention | journal = Molecular Aspects of Medicine | volume = 50 | issue = | pages = 56–87 | date = August 2016 | pmid = 27106402 | doi = 10.1016/j.mam.2016.04.006 | quote = In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is cheap (about US$30–50 per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks. (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions, especially in aged people. }}</ref><ref name="Meta-analytic systematic review September 2015">{{cite journal | vauthors = Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K | display-authors = 6 | title = Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis | journal = Archives of Gerontology and Geriatrics | volume = 61 | issue = 2 | pages = 168–75 | date = September 2015 | pmid = 26169182 | doi = 10.1016/j.archger.2015.06.020 | quote = RESULTS: A total of seven randomized controlled trials were included, in which 147 older adults received HMB intervention and 140 were assigned to control groups. The meta-analysis showed greater muscle mass gain in the intervention groups compared with the control groups (standard mean difference=0.352kg; 95% confidence interval: 0.11, 0.594; Z value=2.85; P=0.004). There were no significant fat mass changes between intervention and control groups (standard mean difference=-0.08kg; 95% confidence interval: -0.32, 0.159; Z value=0.66; P=0.511).<br />CONCLUSION: Beta-hydroxy-beta-methylbutyrate supplementation contributed to preservation of muscle mass in older adults. HMB supplementation may be useful in the prevention of muscle atrophy induced by bed rest or other factors. Further studies are needed to determine the precise effects of HMB on muscle strength and physical function in older adults. }}</ref> A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, [[Muscle#Physiology|muscle function]], and [[Muscle#Strength|muscle strength]] in hypercatabolic disease states such as cancer cachexia.<ref name="Review April 2016" /><ref name="Skeletal muscle crosstalk 2016 review">{{cite journal | vauthors = Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L | title = Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease | journal = Journal of the American Medical Directors Association | volume = 17 | issue = 9 | pages = 789–96 | date = September 2016 | pmid = 27324808 | doi = 10.1016/j.jamda.2016.04.019 | quote = Studies suggest dietary protein and leucine or its metabolite b-hydroxy b-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia.<sup>65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength.<sup>44, 65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84</sup> However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB.<sup>85</sup> Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion.<sup>71, 80</sup> Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease.<sup>65, 72, 83, 86, 87</sup> The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation.<sup>68</sup> ...<br />[http://www.jamda.com/cms/attachment/2060482578/2062671998/gr4_lrg.jpg Figure 4: Treatments for sarcopenia.] It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB. }}</ref><ref name="Nutrition supplements for athletes 2014 review">{{cite journal | vauthors = Mullin GE | title = Nutrition supplements for athletes: potential application to malnutrition | journal = Nutrition in Clinical Practice | volume = 29 | issue = 1 | pages = 146–7 | date = February 2014 | pmid = 24336486 | doi = 10.1177/0884533613516130 | quote = There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted. }}</ref> Based upon a [[meta-analysis]] of seven [[randomized controlled trial]]s that was published in 2015, HMB supplementation has efficacy as a treatment for preserving lean muscle mass in older adults.{{#tag:ref|The estimated standard mean difference [[effect size]] for the increase in muscle mass in the HMB treatment groups relative to controls was {{convert|0.352|kg|lbs}} with a [[95% confidence interval]] of {{convert|0.11|–|0.594|kg|lb}}.<ref name="Meta-analytic systematic review September 2015" /> The studies included in the meta-analysis had durations of 2–12 months and the majority of studies lasted 2–3 months.<ref name="Meta-analytic systematic review September 2015" />|group="note"}}<ref name="Meta-analytic systematic review September 2015" /> More research is needed to determine the precise effects of HMB on muscle strength and function in various populations.<ref name="Meta-analytic systematic review September 2015" /> |
||
Since the absence of muscle-building amino acids can contribute to muscle wasting (that which is torn down must be rebuilt with like material), amino acid therapy may be helpful for regenerating damaged or atrophied muscle tissue. The [[branched-chain amino acids]] ([[leucine]], [[isoleucine]], and [[valine]]) are critical to this process, in addition to [[lysine]] and other amino acids.{{Citation needed|date=January 2019}} |
|||
In severe cases of muscular atrophy, the use of an [[anabolic steroid]] such as [[Metandienone|methandrostenolone]] may be administered to patients as a potential treatment. |
In severe cases of muscular atrophy, the use of an [[anabolic steroid]] such as [[Metandienone|methandrostenolone]] may be administered to patients as a potential treatment although use is limited by side effects. A novel class of drugs, called [[selective androgen receptor modulator]]s, is being investigated with promising results. They would have fewer [[side effects]], while still promoting muscle and bone tissue growth and regeneration. These claims have yet to be confirmed in larger clinical trials.<ref>{{Cite journal|last=Srinath|first=Reshmi|last2=Dobs|first2=Adrian|date=2014|title=Enobosarm (GTx-024, S-22): a potential treatment for cachexia|url=https://www.futuremedicine.com/doi/10.2217/fon.13.273|journal=Future Oncology|language=en|volume=10|issue=2|pages=187–194|doi=10.2217/fon.13.273|issn=1479-6694|via=}}</ref> |
||
A novel class of drugs, called [[selective androgen receptor modulator]]s, is being investigated with promising results. They would have fewer [[side effects]], while still promoting muscle and bone tissue growth and regeneration. These claims are, however, yet to be confirmed in larger clinical trials.{{Citation needed|date=January 2019}} |
|||
One important rehabilitation tool for muscle atrophy includes the use of [[functional electrical stimulation]] to stimulate the muscles. This has seen a large amount of success in the rehabilitation of paraplegic patients.<ref>D.Zhang et al., Functional Electrical Stimulation in Rehabilitation Engineering: A survey, Nenyang technological University, Singapore</ref> |
|||
==Hibernation== |
==Hibernation== |
||
Revision as of 12:35, 20 October 2019
This article needs more reliable medical references for verification or relies too heavily on primary sources. (May 2016) |  |
| Muscle atrophy | |
|---|---|
 | |
| Prisoner of war exhibiting muscle loss as a result of malnutrition | |
| Specialty | Physical Medicine and Rehabilitation |
Muscle atrophy is the loss of skeletal muscle mass that can be caused by immobility, aging, malnutrition, medications, or a wide range of injuries or diseases that impact the musculoskeletal or nervous system. Muscle atrophy leads to muscle weakness and cause disability.
Disuse causes rapid muscle atrophy and often occurs during injury or illness that requires immobilization of a limb or bed rest. Depending on the duration of disuse and the health of the individual, this may be fully reversed with activity. Malnutrition first causes fat loss but may progress to muscle atrophy in prolonged starvation and can be reversed with nutritional therapy. In contrast, cachexia is a wasting syndrome caused by an underlying disease such as cancer that causes dramatic muscle atrophy and cannot be completely reversed with nutritional therapy. Sarcopenia is the muscle atrophy associated with aging and can be slowed by exercise. Finally, diseases of the muscles such as muscular dystrophy or myopathies can cause atrophy, as well as damage to the nervous system such as in spinal cord injury or stroke.
Muscle atrophy results from an imbalance between protein synthesis and protein degradation, although the mechanisms are incompletely understood and are variable depending on the cause. Muscle loss can be quantified with advanced imaging studies but this is not frequently pursued. Treatment depends on the underlying cause but will often include exercise and adequate nutrition. Anabolic agents may have some efficacy but are not often used due to side effects. There are multiple treatments and supplements under investigation but there are currently limited treatment options in clinical practice. Given the implications of muscle atrophy and limited treatment options, minimizing immobility is critical in injury or illness.
Signs and symptoms
The hallmark sign of muscle atrophy is loss of lean muscle mass. This change may be difficult to detect due to obesity, changes in fat mass or edema. Changes in weight, limb or waist circumference are not reliable indicators of muscle mass changes.[1]
The predominant symptom is increased weakness which may result in difficulty or inability in performing physical tasks depending on what muscles are affected. Atrophy of the core or leg muscles may cause difficulty standing from a seated position, walking or climbing stairs and can cause increased falls. Atrophy of the throat muscles may cause difficulty swallowing and diaphragm atrophy can cause difficulty breathing. Muscle atrophy can be asymptomatic and may go undetected until a significant amount of muscle is lost.[2]
Causes
Many diseases and conditions can cause atrophy, including immobility, aging, malnutrition, certain disease states especially with a significant inflammatory component (cancer, congestive heart failure; chronic obstructive pulmonary disease; AIDS, liver disease, etc.), deinnervation, intrinsic muscle disease or medications (such as glucocorticoids).[3]
Immobility
Disuse is a common cause of muscle atrophy and can be local (due to injury or casting) or general (bed-rest). The rate of muscle atrophy from disuse (10-42 days) is approximately 0.5–0.6% of total muscle mass per day although there is considerable variation between people.[4] The elderly are the most vulnerable to dramatic muscle loss with immobility. Much of the established research has investigated prolonged disuse (>10 days), in which the muscle is compromised primarily by declines in muscle protein synthesis rates rather than changes in muscle protein breakdown. There is evidence to suggest that there may be more active protein breakdown during short term immobility (<10 days).[4]
Cachexia
Certain diseases can cause a complex muscle wasting syndrome known as cachexia. It is commonly seen in cancer, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease and AIDS although it is associated with many disease processes, usually with a significant inflammatory component. Cachexia causes ongoing muscle loss that is not entirely reversed with nutritional therapy.[5] The pathophysiology is incompletely understood but inflammatory cytokines are considered to play a central role. In contrast to weight loss from inadequate caloric intake, cachexia causes predominantly muscle loss instead of fat loss and it is not as responsive to nutritional intervention. Cachexia can significantly compromise quality of life and functional status and is associated with poor outcomes.[6][7]
Sarcopenia
Sarcopenia is the degenerative loss of skeletal muscle mass, quality, and strength associated with aging. The rate of muscle loss is dependent on exercise level, co-morbidities, nutrition and other factors. There are many proposed mechanisms of sarcopenia and is considered to be the result of changes in muscle synthesis signalling pathways and gradual failure in the satellite cells which help to regenerate skeletal muscle fibers, but is incompletely understood.
Sarcopenia can lead to reduction in functional status and cause significant disability but is a distinct condition from cachexia although they may co-exist.[7][8] In 2016 an ICD code for sarcopenia was released, contributing to its acceptance as a disease entity.[9]
Intrinsic muscle diseases
Muscle diseases, such as muscular dystrophy, Amylotrophic lateral sclerosis (ALS), or myositis such as inclusion body myositis can cause muscle atrophy.[10]
Central nervous system damage
Damage to neurons in the brain or spinal cord can cause prominent muscle atrophy.This can be localized muscle atrophy and weakness or paresis such as hemiparesis due to stroke or paraplegia due to spinal cord injury.[11] More widespread damage such as in traumatic brain injury or cerebral palsy can cause generalized muscle atrophy.[12]
Peripheral nervous system damage
Injuries or diseases of peripheral nerves supplying specific muscles can also cause muscle atophy. This is seen in nerve injury due to trauma or surgical complication, nerve entrapment, or inherited diseases such as Charcot-Marie-Tooth disease.[13]
Medications
Some medications are known to cause muscle atrophy, usually due to direct effect on muscles. This includes glucocorticoids causing glucocorticoid myopathy[3] or medications toxic to muscle such as doxorubicin.[14]
Endocrinopathies
Disorders of the endocrine system such as Cushing's disease or hypothyroidism are known to cause muscle atrophy.[15]
Pathophysiology
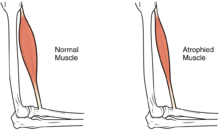
Muscle atrophy occurs by a change in the normal balance between protein synthesis and protein degradation. During atrophy, a down-regulation of protein synthesis pathways occurs, and an activation of protein degradation.[16] The particular protein degradation pathway that seems to be responsible for much of the muscle loss seen in a muscle undergoing atrophy is the ATP-dependent ubiquitin/proteasome pathway. In this system, particular proteins are targeted for destruction by the ligation of at least four copies of a small peptide called ubiquitin onto a substrate protein. When a substrate is thus "poly-ubiquitinated", it is targeted for destruction by the proteasome. Particular enzymes in the ubiquitin/proteasome pathway allow ubiquitination to be directed to some proteins, but not others; specificity is gained by coupling targeted proteins to an "E3 ubiquitin ligase". Each E3 ubiquitin ligase binds to a particular set of substrates, causing their ubiquitination.
Diagnosis
A CT scan can distinguish muscle tissue from other tissues and thereby estimate the amount of muscle tissue in the body.
Fast loss of muscle tissue (relative to normal turnover), can be approximated by the amount of urea in the urine. The equivalent nitrogen content (in grams) of urea (in mmol) can be estimated by the conversion factor 0.028 g/mmol.[17] Furthermore, 1 g of nitrogen is roughly equivalent to 6 g of protein, and 1 g of protein is roughly equivalent to 4 g of muscle tissue. Subsequently, in situations such as muscle wasting, 1 mmol of excessive urea in the urine (as measured by urine volume in litres multiplied by urea concentration in mmol/l) roughly corresponds to a muscle loss of 0.67 g.
Treatment
Treatment approaches include impacting the signaling pathways that induce muscle hypertrophy or slow muscle breakdown as well as optimizing nutritional status.
Exercise is a crucial component to slowing or reversing muscle atrophy. It is still unknown regarding the ideal exercise "dosing." Resistance exercise has been shown to be beneficial in reducing muscle atrophy in older adults.[18][19] In patients who cannot exercise due to physical limitations, include the include the use of functional electrical stimulation to stimulate the muscles. This has seen a large amount of success in the rehabilitation of paraplegic patients.[20]
Adequate calories and protein is crucial to prevent muscle atrophy. Supplementation of protein or branched-chain amino acids, especially leucine, can provide a stimulus for muscle synthesis and inhibit protein breakdown and has been studied for muscle atrophy for sarcopenia and cachexia.[21][22] β-Hydroxy β-methylbutyrate (HMB), a metabolite of leucine which is sold as a dietary supplement, has demonstrated efficacy in preventing the loss of muscle mass in several muscle wasting conditions in humans, particularly sarcopenia.[23][24][25] A growing body of evidence supports the efficacy of HMB as a treatment for reducing, or even reversing, the loss of muscle mass, muscle function, and muscle strength in hypercatabolic disease states such as cancer cachexia.[24][26][27] Based upon a meta-analysis of seven randomized controlled trials that was published in 2015, HMB supplementation has efficacy as a treatment for preserving lean muscle mass in older adults.[note 1][25] More research is needed to determine the precise effects of HMB on muscle strength and function in various populations.[25]
In severe cases of muscular atrophy, the use of an anabolic steroid such as methandrostenolone may be administered to patients as a potential treatment although use is limited by side effects. A novel class of drugs, called selective androgen receptor modulators, is being investigated with promising results. They would have fewer side effects, while still promoting muscle and bone tissue growth and regeneration. These claims have yet to be confirmed in larger clinical trials.[28]
Hibernation
Inactivity and starvation in mammals lead to atrophy of skeletal muscle, accompanied by a smaller number and size of the muscle cells as well as lower protein content.[29] In humans, prolonged periods of immobilization, as in the cases of bed rest or astronauts flying in space, are known to result in muscle weakening and atrophy. Such consequences are also noted in small hibernating mammals like the golden-mantled ground squirrels and brown bats.[30]
Bears are an exception to this rule; species in the family Ursidae are famous for their ability to survive unfavorable environmental conditions of low temperatures and limited nutrition availability during winter by means of hibernation. During that time, bears go through a series of physiological, morphological, and behavioral changes.[31] Their ability to maintain skeletal muscle number and size during disuse is of significant importance.
During hibernation, bears spend 4-7 months of inactivity and anorexia without undergoing muscle atrophy and protein loss.[30] A few known factors contribute to the sustaining of muscle tissue. During the summer, bears take advantage of the nutrition availability and accumulate muscle protein. The protein balance at time of dormancy is also maintained by lower levels of protein breakdown during the winter.[30] At times of immobility, muscle wasting in bears is also suppressed by a proteolytic inhibitor that is released in circulation.[29] Another factor that contributes to the sustaining of muscle strength in hibernating bears is the occurrence of periodic voluntary contractions and involuntary contractions from shivering during torpor.[32] The three to four daily episodes of muscle activity are responsible for the maintenance of muscle strength and responsiveness in bears during hibernation.[32]
See also
Notes
- ^ The estimated standard mean difference effect size for the increase in muscle mass in the HMB treatment groups relative to controls was 0.352 kilograms (0.78 lb) with a 95% confidence interval of 0.11–0.594 kilograms (0.24–1.31 lb).[25] The studies included in the meta-analysis had durations of 2–12 months and the majority of studies lasted 2–3 months.[25]
References
- ^ Dev R (January 2019). "Measuring cachexia-diagnostic criteria". Annals of Palliative Medicine. 8 (1): 24–32. doi:10.21037/apm.2018.08.07. PMID 30525765.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cretoiu SM, Zugravu CA (2018). Xiao J (ed.). "Nutritional Considerations in Preventing Muscle Atrophy". Advances in Experimental Medicine and Biology. 1088. Springer Singapore: 497–528. doi:10.1007/978-981-13-1435-3_23. ISBN 9789811314346. PMID 30390267.
- ^ a b Seene T (July 1994). "Turnover of skeletal muscle contractile proteins in glucocorticoid myopathy". The Journal of Steroid Biochemistry and Molecular Biology. 50 (1–2): 1–4. doi:10.1016/0960-0760(94)90165-1. PMID 8049126.
- ^ a b Wall BT, Dirks ML, van Loon LJ (September 2013). "Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia". Ageing Research Reviews. 12 (4): 898–906. doi:10.1016/j.arr.2013.07.003. PMID 23948422.
- ^ Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. (December 2008). "Cachexia: a new definition". Clinical Nutrition. 27 (6): 793–9. doi:10.1016/j.clnu.2008.06.013. PMID 18718696.
- ^ Morley JE, Thomas DR, Wilson MM (April 2006). "Cachexia: pathophysiology and clinical relevance". The American Journal of Clinical Nutrition. 83 (4): 735–43. doi:10.1093/ajcn/83.4.735. PMID 16600922.
- ^ a b Peterson SJ, Mozer M (February 2017). "Differentiating Sarcopenia and Cachexia Among Patients With Cancer". Nutrition in Clinical Practice. 32 (1): 30–39. doi:10.1177/0884533616680354. PMID 28124947.
- ^ Marcell TJ (October 2003). "Sarcopenia: causes, consequences, and preventions". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 58 (10): M911-6. doi:10.1093/gerona/58.10.m911. PMID 14570858.
- ^ Anker SD, Morley JE, von Haehling S (December 2016). "Welcome to the ICD-10 code for sarcopenia". Journal of Cachexia, Sarcopenia and Muscle. 7 (5): 512–514. doi:10.1002/jcsm.12147. PMC 5114626. PMID 27891296.
- ^ Powers SK, Lynch GS, Murphy KT, Reid MB, Zijdewind I (November 2016). "Disease-Induced Skeletal Muscle Atrophy and Fatigue". Medicine and Science in Sports and Exercise. 48 (11): 2307–2319. doi:10.1249/MSS.0000000000000975. PMC 5069191. PMID 27128663.
- ^ O'Brien LC, Gorgey AS (October 2016). "Skeletal muscle mitochondrial health and spinal cord injury". World Journal of Orthopedics. 7 (10): 628–637. doi:10.5312/wjo.v7.i10.628. PMC 5065669. PMID 27795944.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Verschuren O, Smorenburg AR, Luiking Y, Bell K, Barber L, Peterson MD (June 2018). "Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: a narrative review of the literature". Journal of Cachexia, Sarcopenia and Muscle. 9 (3): 453–464. doi:10.1002/jcsm.12287. PMC 5989853. PMID 29392922.
- ^ Wong A, Pomerantz JH (March 2019). "The Role of Muscle Stem Cells in Regeneration and Recovery after Denervation: A Review". Plastic and Reconstructive Surgery. 143 (3): 779–788. doi:10.1097/PRS.0000000000005370. PMID 30817650.
- ^ Hiensch AE, Bolam KA, Mijwel S, Jeneson JA, Huitema AD, Kranenburg O, et al. (October 2019). "Doxorubicin-induced skeletal muscle atrophy: elucidating the underlying molecular pathways". Acta Physiologica: e13400. doi:10.1111/apha.13400. PMID 31600860.
- ^ Martín AI, Priego T, López-Calderón A (2018). Xiao J (ed.). "Hormones and Muscle Atrophy". Advances in Experimental Medicine and Biology. 1088. Springer Singapore: 207–233. doi:10.1007/978-981-13-1435-3_9. ISBN 9789811314346. PMID 30390253.
- ^ Sandri M (June 2008). "Signaling in muscle atrophy and hypertrophy". Physiology. 23. Bethesda, Md.: 160–70. doi:10.1152/physiol.00041.2007. PMID 18556469.
- ^ Section 1.9.2 (page 76) in: Bishop, Jacki; Briony, Thomas (2007). Manual of Dietetic Practice. Wiley-Blackwell. ISBN 978-1-4051-3525-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sayer, Avan Aihie (2014). "Sarcopenia the new geriatric giant: time to translate research findings into clinical practice". Age and Ageing. 43 (6): 736–737. doi:10.1093/ageing/afu118. ISSN 1468-2834. PMID 25227204.
- ^ Liu, Chiung-Ju; Latham, Nancy K. (2009-07-08). "Progressive resistance strength training for improving physical function in older adults". The Cochrane Database of Systematic Reviews (3): CD002759. doi:10.1002/14651858.CD002759.pub2. ISSN 1469-493X. PMC 4324332. PMID 19588334.
- ^ D.Zhang et al., Functional Electrical Stimulation in Rehabilitation Engineering: A survey, Nenyang technological University, Singapore
- ^ Sakamoto, N.; Enomoto, N.; Kurosaki, M.; Asahina, Y.; Maekawa, S.; Koizumi, K.; Sakuma, I.; Murakami, T.; Marumo, F.; Sato, C. (1995). "Comparison of the hypervariable region of hepatitis C virus genomes in plasma and liver". Journal of Medical Virology. 46 (1): 7–11. doi:10.1002/jmv.1890460103. ISSN 0146-6615. PMID 7623010.
- ^ Argilés, Josep M.; Campos, Nefertiti; Lopez-Pedrosa, José M.; Rueda, Ricardo; Rodriguez-Mañas, Leocadio (2016). "Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease". Journal of the American Medical Directors Association. 17 (9): 789–796. doi:10.1016/j.jamda.2016.04.019.
- ^ Phillips SM (July 2015). "Nutritional supplements in support of resistance exercise to counter age-related sarcopenia". Advances in Nutrition. 6 (4): 452–60. doi:10.3945/an.115.008367. PMC 4496741. PMID 26178029.
- ^ a b Brioche T, Pagano AF, Py G, Chopard A (August 2016). "Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention". Molecular Aspects of Medicine. 50: 56–87. doi:10.1016/j.mam.2016.04.006. PMID 27106402.
In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is cheap (about US$30–50 per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks. (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions, especially in aged people.
- ^ a b c d e Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, et al. (September 2015). "Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis". Archives of Gerontology and Geriatrics. 61 (2): 168–75. doi:10.1016/j.archger.2015.06.020. PMID 26169182.
RESULTS: A total of seven randomized controlled trials were included, in which 147 older adults received HMB intervention and 140 were assigned to control groups. The meta-analysis showed greater muscle mass gain in the intervention groups compared with the control groups (standard mean difference=0.352kg; 95% confidence interval: 0.11, 0.594; Z value=2.85; P=0.004). There were no significant fat mass changes between intervention and control groups (standard mean difference=-0.08kg; 95% confidence interval: -0.32, 0.159; Z value=0.66; P=0.511).
CONCLUSION: Beta-hydroxy-beta-methylbutyrate supplementation contributed to preservation of muscle mass in older adults. HMB supplementation may be useful in the prevention of muscle atrophy induced by bed rest or other factors. Further studies are needed to determine the precise effects of HMB on muscle strength and physical function in older adults. - ^ Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L (September 2016). "Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease". Journal of the American Medical Directors Association. 17 (9): 789–96. doi:10.1016/j.jamda.2016.04.019. PMID 27324808.
Studies suggest dietary protein and leucine or its metabolite b-hydroxy b-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia.65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84 A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength.44, 65, 72, 76, 77, 78, 79, 80, 81, 82, 83, 84 However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB.85 Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion.71, 80 Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease.65, 72, 83, 86, 87 The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation.68 ...
Figure 4: Treatments for sarcopenia. It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB.{{cite journal}}: External link in|quote= - ^ Mullin GE (February 2014). "Nutrition supplements for athletes: potential application to malnutrition". Nutrition in Clinical Practice. 29 (1): 146–7. doi:10.1177/0884533613516130. PMID 24336486.
There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted.
- ^ Srinath, Reshmi; Dobs, Adrian (2014). "Enobosarm (GTx-024, S-22): a potential treatment for cachexia". Future Oncology. 10 (2): 187–194. doi:10.2217/fon.13.273. ISSN 1479-6694.
- ^ a b Fuster G, Busquets S, Almendro V, López-Soriano FJ, Argilés JM (October 2007). "Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy?". Clinical Nutrition. 26 (5): 658–61. doi:10.1016/j.clnu.2007.07.003. PMID 17904252.
- ^ a b c Lohuis TD, Harlow HJ, Beck TD (May 2007). "Hibernating black bears (Ursus americanus) experience skeletal muscle protein balance during winter anorexia". Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 147 (1): 20–8. doi:10.1016/j.cbpb.2006.12.020. PMID 17307375.
- ^ Carey HV, Andrews MT, Martin SL (October 2003). "Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature". Physiological Reviews. 83 (4): 1153–81. doi:10.1152/physrev.00008.2003. PMID 14506303.
- ^ a b Harlow HJ, Lohuis T, Anderson-Sprecher RC, Beck TD (2004). "Body Surface Temperature Of Hibernating Black Bears May Be Related To Periodic Muscle Activity". Journal of Mammalogy. 85 (3): 414–419. doi:10.1644/1545-1542(2004)085<0414:BSTOHB>2.0.CO;2.
External links
 Media related to Muscle atrophy at Wikimedia Commons
Media related to Muscle atrophy at Wikimedia Commons- Muscular atrophy at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
