Marine viruses: Difference between revisions
Epipelagic (talk | contribs) add images plus copy from viral shunt |
Epipelagic (talk | contribs) |
||
| Line 77: | Line 77: | ||
Viruses are an important natural means of [[Horizontal gene transfer|transferring genes]] between different species, which increases [[genetic diversity]] and drives evolution.<ref name="Canchaya">{{vcite journal|author=Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H|title=Phage as agents of lateral gene transfer|journal=Current Opinion in Microbiology |volume=6 |issue=4 |pages=417–24 |year=2003 |pmid=12941415|doi=10.1016/S1369-5274(03)00086-9}}</ref> It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the [[Last universal ancestor|last universal common ancestor]] of life on Earth.<ref name="pmid11536914">{{vcite journal |author=Forterre P, Philippe H |title=The last universal common ancestor (LUCA), simple or complex? |journal=The Biological Bulletin |volume=196 |issue=3 |pages=373–5; discussion 375–7 |year=1999 |pmid=11536914 |doi= 10.2307/1542973}}</ref> Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.<ref name="pmid17853907" /> |
Viruses are an important natural means of [[Horizontal gene transfer|transferring genes]] between different species, which increases [[genetic diversity]] and drives evolution.<ref name="Canchaya">{{vcite journal|author=Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H|title=Phage as agents of lateral gene transfer|journal=Current Opinion in Microbiology |volume=6 |issue=4 |pages=417–24 |year=2003 |pmid=12941415|doi=10.1016/S1369-5274(03)00086-9}}</ref> It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the [[Last universal ancestor|last universal common ancestor]] of life on Earth.<ref name="pmid11536914">{{vcite journal |author=Forterre P, Philippe H |title=The last universal common ancestor (LUCA), simple or complex? |journal=The Biological Bulletin |volume=196 |issue=3 |pages=373–5; discussion 375–7 |year=1999 |pmid=11536914 |doi= 10.2307/1542973}}</ref> Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.<ref name="pmid17853907" /> |
||
[[Virus]]es are part of the [[hydrothermal vent microbial community]] and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.<ref>{{Cite journal|last=Anderson|first=Rika E.|last2=Brazelton|first2=William J.|last3=Baross|first3=John A.|date=2011|title=Is the genetic landscape of the deep subsurface biosphere affected by viruses?|journal=Frontiers in Microbiology|volume=2|pages=219|doi=10.3389/fmicb.2011.00219|issn=1664-302X|pmc=3211056|pmid=22084639}}</ref> Viruses are the most abundant life in the ocean, harboring the greatest reservoir of genetic diversity.<ref>{{Cite journal|last=Suttle|first=Curtis A.|date=September 2005|title=Viruses in the sea|journal=Nature|volume=437|issue=7057|pages=356–361|doi=10.1038/nature04160|pmid=16163346|issn=0028-0836|bibcode=2005Natur.437..356S}}</ref> As their infections are often fatal, they constitute a significant source of mortality and thus have widespread influence on biological oceanographic processes, [[evolution]] and [[Geochemical cycle#Differentiating biogeochemical cycles|biogeochemical cycling]] within the ocean.<ref>{{Cite journal|last=Suttle|first=Curtis A.|date=October 2007|title=Marine viruses — major players in the global ecosystem|journal=Nature Reviews Microbiology|language=En|volume=5|issue=10|pages=801–812|doi=10.1038/nrmicro1750|pmid=17853907|issn=1740-1526}}</ref> Evidence has been found however to indicate that viruses found in vent habitats have adopted a more [[Mutualism (biology)|mutualistic]] than [[Parasitism|parasitic]] evolutionary strategy in order to survive the extreme and volatile environment they exist in.<ref name=":17">{{Cite journal|last=Anderson|first=Rika E.|last2=Sogin|first2=Mitchell L.|last3=Baross|first3=John A.|date=2014-10-03|title=Evolutionary Strategies of Viruses, Bacteria and Archaea in Hydrothermal Vent Ecosystems Revealed through Metagenomics|journal=PLOS ONE|volume=9|issue=10|pages=e109696|doi=10.1371/journal.pone.0109696|issn=1932-6203|pmc=4184897|pmid=25279954|bibcode=2014PLoSO...9j9696A}}</ref> Deep-sea hydrothermal vents were found to have high numbers of viruses indicating high viral production.<ref name=":18">{{Cite journal|last=Ortmann|first=Alice C.|last2=Suttle|first2=Curtis A.|date=August 2005|title=High abundances of viruses in a deep-sea hydrothermal vent system indicates viral mediated microbial mortality|journal=Deep Sea Research Part I: Oceanographic Research Papers|volume=52|issue=8|pages=1515–1527|doi=10.1016/j.dsr.2005.04.002|issn=0967-0637|bibcode=2005DSRI...52.1515O}}</ref> Like in other marine environments, [[Viruses and deep-sea hydrothermal vents|deep-sea hydrothermal viruses]] affect abundance and diversity of [[prokaryote]]s and therefore impact microbial biogeochemical cycling by [[Lysis|lysing]] their hosts to replicate.<ref>{{Cite journal|authorlink=Mya Breitbart|last=Breitbart|first=Mya|date=2012-01-15|title=Marine Viruses: Truth or Dare|journal=Annual Review of Marine Science|language=en|volume=4|issue=1|pages=425–448|doi=10.1146/annurev-marine-120709-142805|pmid=22457982|issn=1941-1405|bibcode=2012ARMS....4..425B}}</ref> However, in contrast to their role as a source of mortality and population control, viruses have also been postulated to enhance survival of prokaryotes in extreme environments, acting as reservoirs of genetic information. The interactions of the virosphere with microorganisms under environmental stresses is therefore thought to aide microorganism survival through dispersal of host genes through [[Horizontal gene transfer in evolution|horizontal gene transfer]].<ref>{{Cite journal|last=Goldenfeld|first=Nigel|last2=Woese|first2=Carl|date=January 2007|title=Biology's next revolution|journal=Nature|volume=445|issue=7126|pages=369|doi=10.1038/445369a|pmid=17251963|issn=0028-0836|arxiv=q-b |
|||
{{clear}} |
{{clear}} |
||
Revision as of 09:57, 10 May 2020
including viral infection of bacteria, phytoplankton and fish[1]
A virus is a small infectious agent that replicates only inside the living cells of other organisms. Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea.[2]
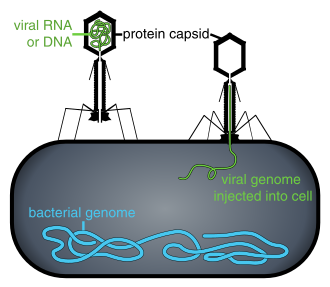
When not inside an infected cell or in the process of infecting a cell, viruses exist in the form of independent particles. These viral particles, also known as virions, consist of two or three parts: (i) the genetic material (genome) made from either DNA or RNA, long molecules that carry genetic information; (ii) a protein coat called the capsid, which surrounds and protects the genetic material; and in some cases (iii) an envelope of lipids that surrounds the protein coat when they are outside a cell. The shapes of these virus particles range from simple helical and icosahedral forms for some virus species to more complex structures for others. Most virus species have virions that are too small to be seen with an optical microscope. The average virion is about one one-hundredth the size of the average bacterium.
Background
Viruses are found wherever there is life and have probably existed since living cells first evolved.[3] The origins of viruses in the evolutionary history of life are unclear because they do not form fossils. molecular techniques have been used to compare the DNA or RNA of viruses and are a useful means of investigating how they arose.[4] Some viruses may have evolved from plasmids—pieces of DNA that can move between cells—while others may have evolved from bacteria. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity.[5]
Viruses are now recognised as ancient and as having origins that pre-date the divergence of life into the three domains.[6]
Opinions differ on whether viruses are a form of life or organic structures that interact with living organisms.[7] They are considered by some to be a life form, because they carry genetic material, reproduce by creating multiple copies of themselves through self-assembly, and evolve through natural selection. However they lack key characteristics such as a cellular structure generally considered necessary to count as life. Because they possess some but not all such qualities, viruses have been described as replicators[8] and as "organisms at the edge of life".[9]
Marine phages

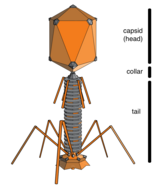
Bacteriophages, often just called phages, are viruses that parasite bacteria and archaea. Marine phages parasite marine bacteria and archaea, such as cyanobacteria.[10] They are a common and diverse group of viruses and are the most abundant biological entity in marine environments, because their hosts, bacteria, are typically the numerically dominant cellular life in the sea. Generally there are about 1 million to 10 million viruses in each mL of seawater, or about ten times more double-stranded DNA viruses than there are cellular organisms,[11][12] although estimates of viral abundance in seawater can vary over a wide range.[13][14] For a long time, tailed phages of the order Caudovirales seemed to dominate marine ecosystems in number and diversity of organisms.[10]

However, as a result of more resent research, non-tailed viruses appear to be dominant in multiple depths and oceanic regions, followed by the Caudovirales families of myoviruses, podoviruses, and siphoviruses.[15] Phages belonging to the families Corticoviridae,[16] Inoviridae,[17] Microviridae,[18] and Autolykiviridae[19][20][21][22] are also known to infect diverse marine bacteria.
There are also archaean viruses which replicate within archaea: these are double-stranded DNA viruses with unusual and sometimes unique shapes.[23][24] These viruses have been studied in most detail in the thermophilic archaea, particularly the orders Sulfolobales and Thermoproteales.[25]
Role of marine viruses

Microorganisms make up about 70% of the marine biomass.[27] It is estimated viruses kill 20% of this biomass each day and that there are 15 times as many viruses in the oceans as there are bacteria and archaea. Viruses are the main agents responsible for the rapid destruction of harmful algal blooms,[28] which often kill other marine life.[29] The number of viruses in the oceans decreases further offshore and deeper into the water, where there are fewer host organisms.[30]
Viruses are an important natural means of transferring genes between different species, which increases genetic diversity and drives evolution.[5] It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the last universal common ancestor of life on Earth.[31] Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.[30]
Viruses are part of the hydrothermal vent microbial community and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.[32] Viruses are the most abundant life in the ocean, harboring the greatest reservoir of genetic diversity.[33] As their infections are often fatal, they constitute a significant source of mortality and thus have widespread influence on biological oceanographic processes, evolution and biogeochemical cycling within the ocean.[34] Evidence has been found however to indicate that viruses found in vent habitats have adopted a more mutualistic than parasitic evolutionary strategy in order to survive the extreme and volatile environment they exist in.[35] Deep-sea hydrothermal vents were found to have high numbers of viruses indicating high viral production.[36] Like in other marine environments, deep-sea hydrothermal viruses affect abundance and diversity of prokaryotes and therefore impact microbial biogeochemical cycling by lysing their hosts to replicate.[37] However, in contrast to their role as a source of mortality and population control, viruses have also been postulated to enhance survival of prokaryotes in extreme environments, acting as reservoirs of genetic information. The interactions of the virosphere with microorganisms under environmental stresses is therefore thought to aide microorganism survival through dispersal of host genes through horizontal gene transfer.Cite error: A <ref> tag is missing the closing </ref> (see the help page). The DOM recycled by the viral shunt pathway is comparable to the amount generated by the other main sources of marine DOM.[38]
Viruses can easily infect microorganisms in the microbial loop due to their relative abundance compared to microbes.[39] Prokaryotic and eukaryotic mortality contribute to carbon nutrient recycling through cell lysis. There is evidence as well of nitrogen (specifically ammonium) regeneration. This nutrient recycling helps stimulates microbial growth.[40] As much as 25% of the primary production from phytoplankton in the global oceans may be recycled within the microbial loop through viral shunting.[41]
Giant marine viruses

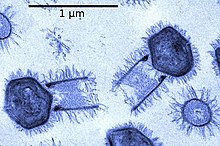
Viruses normally range in length from about 20 to 300 nanometers. This can be contrasted with the length of bacteria, which starts at about 400 nanometers. There are also giant viruses, often called giruses, typically about 1000 nanometers (one micron) in length. All giant viruses belongto phylum Nucleocytoviricota (NCLDV), together with poxviruses. The largest known of these is Tupanvirus. This genus of giant virus was discovered in 2018 in the deep ocean as well as a soda lake, and can reach up to 2.3 microns in total length.[42]
The discovery and subsequent characterization of giant viruses has triggered some debate concerning their evolutionary origins. The two main hypotheses for their origin are that either they evolved from small viruses, picking up DNA from host organisms, or that they evolved from very complicated organisms into the current form which is not self-sufficient for reproduction.[43] What sort of complicated organism giant viruses might have diverged from is also a topic of debate. One proposal is that the origin point actually represents a fourth domain of life,[44][45] but this has been largely discounted.[46][47]
References
- ^ Middelboe, M.; Brussaard, C. (2017). "Marine viruses: key players in marine ecosystems". Viruses. 9 (10): 302. doi:10.3390/v9100302. PMID 29057790.
- ^ Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biology Direct. 2006;1:29. doi:10.1186/1745-6150-1-29. PMID 16984643.
- ^ Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Research. 2006;117(1):156–84. doi:10.1016/j.virusres.2006.01.009. PMID 16494962.
- ^ Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. Journal of Virology. 2010;84(19):9733–48. doi:10.1128/JVI.00694-10. PMID 20660197.
- ^ a b Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H. Phage as agents of lateral gene transfer. Current Opinion in Microbiology. 2003;6(4):417–24. doi:10.1016/S1369-5274(03)00086-9. PMID 12941415.
- ^ Mahy WJ & Van Regenmortel MHV (eds). Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 28.
- ^ Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question. Studies in History and Philosophy of Biological and Biomedical Sciences. 2016. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- ^ Koonin, E. V.; Starokadomskyy, P. (7 March 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Biological and Biomedical Sciences. 59: 125–34. doi:10.1016/j.shpsc.2016.02.016. PMC 5406846. PMID 26965225.
- ^ Rybicki, EP. The classification of organisms at the edge of life, or problems with virus systematics. South African Journal of Science. 1990;86:182–186.
- ^ a b Mann, NH (2005-05-17). "The Third Age of Phage". PLOS Biology. 3 (5): 753–755. doi:10.1371/journal.pbio.0030182. PMC 1110918. PMID 15884981.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews. 2000;64(1):69–114. doi:10.1128/MMBR.64.1.69-114.2000. PMID 10704475.
- ^ Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi:10.1038/nature04160. PMID 16163346.
- ^ Bergh O, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340(6233):467–8. doi:10.1038/340467a0. PMID 2755508. Bibcode:1989Natur.340..467B.
- ^ Wigington CH, Sonderegger D, Brussaard CPD, Buchan A, Finke JF, Fuhrman JA, Lennon JT, Middelboe M, Suttle CA, Stock C, Wilson WH, Wommack KE, Wilhelm SW, Weitz JS. Re-examination of the relationship between marine virus and microbial cell abundances. Nature Microbiology. 2016;1:15024. doi:10.1038/nmicrobiol.2015.24. PMID 27572161.
- ^ Brum JR, Schenck RO, Sullivan MB (September 2013). "Global morphological analysis of marine viruses shows minimal regional variation and dominance of non-tailed viruses". The ISME Journal. 7 (9): 1738–51. doi:10.1038/ismej.2013.67. PMC 3749506. PMID 23635867.
- ^ Krupovic M, Bamford DH (2007). "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria". BMC Genomics. 8: 236. doi:10.1186/1471-2164-8-236. PMC 1950889. PMID 17634101.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Xue H, Xu Y, Boucher Y, Polz MF (2012). "High frequency of a novel filamentous phage, VCY φ, within an environmental Vibrio cholerae population". Applied and Environmental Microbiology. 78 (1): 28–33. doi:10.1128/AEM.06297-11. PMC 3255608. PMID 22020507.
- ^ Roux S, Krupovic M, Poulet A, Debroas D, Enault F (2012). "Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads". PLOS ONE. 7 (7): e40418. Bibcode:2012PLoSO...740418R. doi:10.1371/journal.pone.0040418. PMC 3394797. PMID 22808158.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kathryn M. Kauffman, Fatima A. Hussain, Joy Yang, Philip Arevalo, Julia M. Brown, William K. Chang, David VanInsberghe, Joseph Elsherbini, Radhey S. Sharma, Michael B. Cutler, Libusha Kelly, Martin F. Polz: A major lineage of non-tailed dsDNA viruses as unrecognized killers of marine bacteria, in: Nature vol. 554, pp. 118–122, 24 January 2018, doi:10.1038/nature25474
- ^ Scientists Find New Type of Virus in World’s Oceans: Autolykiviridae, on: sci-news, 25 January 2018
- ^ Never-Before-Seen Viruses With Weird DNA Were Just Discovered in The Ocean, on: sciencealert, 25 January 2018
- ^ NCBI: Autolykiviridae (family) – unclassified dsDNA viruses
- ^ Lawrence CM, Menon S, Eilers BJ, et al.. Structural and functional studies of archaeal viruses. Journal of Biological Chemistry. 2009;284(19):12599–603. doi:10.1074/jbc.R800078200. PMID 19158076.
- ^ Prangishvili D, Forterre P, Garrett RA. Viruses of the Archaea: a unifying view. Nature Reviews Microbiology. 2006;4(11):837–48. doi:10.1038/nrmicro1527. PMID 17041631.
- ^ Prangishvili D, Garrett RA. Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses. Biochemical Society Transactions. 2004;32(Pt 2):204–8. doi:10.1042/BST0320204. PMID 15046572.
- ^ Heinrichs, M.E., Mori, C. and Dlugosch, L. (2020) "Complex Interactions Between Aquatic Organisms and Their Chemical Environment Elucidated from Different Perspectives". In: YOUMARES 9-The Oceans: Our Research, Our Future , pages 279–297. Springer. doi:10.1007/978-3-030-20389-4_15.
- ^ Bar-On, YM; Phillips, R; Milo, R (2018). "The biomass distribution on Earth". PNAS. 115 (25): 6506–6511. doi:10.1073/pnas.1711842115. PMC 6016768. PMID 29784790.
- ^ Suttle CA. Viruses in the sea. Nature. 2005;437(7057):356–61. doi:10.1038/nature04160. PMID 16163346. Bibcode:2005Natur.437..356S.
- ^ www.cdc.gov. Harmful Algal Blooms: Red Tide: Home [Retrieved 2014-12-19].
- ^ a b Suttle CA. Marine viruses—major players in the global ecosystem. Nature Reviews Microbiology. 2007;5(10):801–12. doi:10.1038/nrmicro1750. PMID 17853907.
- ^ Forterre P, Philippe H. The last universal common ancestor (LUCA), simple or complex?. The Biological Bulletin. 1999;196(3):373–5; discussion 375–7. doi:10.2307/1542973. PMID 11536914.
- ^ Anderson, Rika E.; Brazelton, William J.; Baross, John A. (2011). "Is the genetic landscape of the deep subsurface biosphere affected by viruses?". Frontiers in Microbiology. 2: 219. doi:10.3389/fmicb.2011.00219. ISSN 1664-302X. PMC 3211056. PMID 22084639.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Suttle, Curtis A. (September 2005). "Viruses in the sea". Nature. 437 (7057): 356–361. Bibcode:2005Natur.437..356S. doi:10.1038/nature04160. ISSN 0028-0836. PMID 16163346.
- ^ Suttle, Curtis A. (October 2007). "Marine viruses — major players in the global ecosystem". Nature Reviews Microbiology. 5 (10): 801–812. doi:10.1038/nrmicro1750. ISSN 1740-1526. PMID 17853907.
- ^ Anderson, Rika E.; Sogin, Mitchell L.; Baross, John A. (2014-10-03). "Evolutionary Strategies of Viruses, Bacteria and Archaea in Hydrothermal Vent Ecosystems Revealed through Metagenomics". PLOS ONE. 9 (10): e109696. Bibcode:2014PLoSO...9j9696A. doi:10.1371/journal.pone.0109696. ISSN 1932-6203. PMC 4184897. PMID 25279954.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ortmann, Alice C.; Suttle, Curtis A. (August 2005). "High abundances of viruses in a deep-sea hydrothermal vent system indicates viral mediated microbial mortality". Deep Sea Research Part I: Oceanographic Research Papers. 52 (8): 1515–1527. Bibcode:2005DSRI...52.1515O. doi:10.1016/j.dsr.2005.04.002. ISSN 0967-0637.
- ^ Breitbart, Mya (2012-01-15). "Marine Viruses: Truth or Dare". Annual Review of Marine Science. 4 (1): 425–448. Bibcode:2012ARMS....4..425B. doi:10.1146/annurev-marine-120709-142805. ISSN 1941-1405. PMID 22457982.
- ^ Robinson, Carol, and Nagappa Ramaiah. "Microbial heterotrophic metabolic rates constrain the microbial carbon pump." The American Association for the Advancement of Science, 2011.
- ^ Fuhrman, Jed A. (1999). "Marine viruses and their biogeochemical and ecological effects". Nature. 399 (6736): 541–548. Bibcode:1999Natur.399..541F. doi:10.1038/21119. ISSN 0028-0836. PMID 10376593.
- ^ Tsai, An-Yi, Gwo-Ching Gong, and Yu-Wen Huang. "Importance of the Viral Shunt in Nitrogen Cycling in Synechococcus Spp. Growth in Subtropical Western Pacific Coastal Waters." Terrestrial, Atmospheric & Oceanic Sciences25.6 (2014).
- ^ Wilhelm, Steven W.; Suttle, Curtis A. (1999). "Viruses and nutrient cycles in the sea: viruses play critical roles in the structure and function of aquatic food webs". BioScience. 49 (10): 781–788. doi:10.2307/1313569. JSTOR 1313569.
- ^ Abrahão, Jônatas; Silva, Lorena; Silva, Ludmila Santos; Khalil, Jacques Yaacoub Bou; Rodrigues, Rodrigo; Arantes, Thalita; Assis, Felipe; Boratto, Paulo; Andrade, Miguel; Kroon, Erna Geessien; Ribeiro, Bergmann; Bergier, Ivan; Seligmann, Herve; Ghigo, Eric; Colson, Philippe; Levasseur, Anthony; Kroemer, Guido; Raoult, Didier; Scola, Bernard La (27 February 2018). "Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere". Nature Communications. 9 (1): 749. Bibcode:2018NatCo...9..749A. doi:10.1038/s41467-018-03168-1. PMC 5829246. PMID 29487281.
- ^ Bichell, Rae Ellen. "In Giant Virus Genes, Hints About Their Mysterious Origin". All Things Considered.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Van Etten, James L. (July–August 2011). "Giant Viruses". American Scientist. 99 (4): 304–311. doi:10.1511/2011.91.304.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Legendre M, Arslan D, Abergel C, Claverie JM (January 2012). "Genomics of Megavirus and the elusive fourth domain of Life". Communicative & Integrative Biology. 5 (1): 102–6. doi:10.4161/cib.18624. PMC 3291303. PMID 22482024.
- ^ Schulz F, Yutin N, Ivanova NN, Ortega DR, Lee TK, Vierheilig J, Daims H, Horn M, Wagner M, Jensen GJ, Kyrpides NC, Koonin EV, Woyke T (April 2017). "Giant viruses with an expanded complement of translation system components" (PDF). Science. 356 (6333): 82–85. Bibcode:2017Sci...356...82S. doi:10.1126/science.aal4657. PMID 28386012.
- ^ Bäckström D, Yutin N, Jørgensen SL, Dharamshi J, Homa F, Zaremba-Niedwiedzka K, Spang A, Wolf YI, Koonin EV, Ettema TJ (March 2019). "Virus Genomes from Deep Sea Sediments Expand the Ocean Megavirome and Support Independent Origins of Viral Gigantism". mBio. 10 (2): e02497-02418. doi:10.1128/mBio.02497-18. PMC 6401483. PMID 30837339.