Synthetic molecular motor: Difference between revisions
m added wikilinks to Boston College and Ben Feringa |
Citation bot (talk | contribs) Add: url, s2cid, pmc, pages, volume, doi, pmid, issue, author pars. 1-1. Removed URL that duplicated unique identifier. Removed parameters. Formatted dashes. Some additions/deletions were actually parameter name changes. | You can use this bot yourself. Report bugs here. | Suggested by SemperIocundus | via #UCB_webform |
||
| Line 1: | Line 1: | ||
{{About|man-made molecular motors|the naturally occurring protein motors|Molecular motor}} |
{{About|man-made molecular motors|the naturally occurring protein motors|Molecular motor}} |
||
'''Synthetic molecular motors''' are [[molecular machine]]s capable of continuous directional rotation under an energy input.<ref>{{cite journal | doi = 10.1039/C7CS00245A | volume=46 | title=Artificial molecular motors | year=2017 | journal=Chemical Society Reviews | pages=2592–2621 | last1 = Kassem | first1 = Salma | last2 = van Leeuwen | first2 = Thomas | last3 = Lubbe | first3 = Anouk S. | last4 = Wilson | first4 = Miriam R. | last5 = Feringa | first5 = Ben L. | last6 = Leigh | first6 = David A.}}</ref> Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via [[protein dynamics]]), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors. |
'''Synthetic molecular motors''' are [[molecular machine]]s capable of continuous directional rotation under an energy input.<ref>{{cite journal | doi = 10.1039/C7CS00245A | volume=46 | title=Artificial molecular motors | year=2017 | journal=Chemical Society Reviews | pages=2592–2621 | last1 = Kassem | first1 = Salma | last2 = van Leeuwen | first2 = Thomas | last3 = Lubbe | first3 = Anouk S. | last4 = Wilson | first4 = Miriam R. | last5 = Feringa | first5 = Ben L. | last6 = Leigh | first6 = David A.| issue=9 | pmid=28426052 }}</ref> Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via [[protein dynamics]]), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors. |
||
[[File:MD rotor 250K 1ns.gif|thumb|Molecular dynamics simulation of a synthetic molecular rotor composed of three molecules in a nanopore (outer diameter 6.7 nm) at 250 K.<ref>{{cite journal|title=Topological Dynamics in Supramolecular Rotors|journal=Nano Letters|volume=148|pages=4461–4468|year=2014|last1=Palma|first1=C.-A.|last2=Björk|first2=J.|last3=Rao|first3=F.|last4=Kühne|first4=D.|last5=Klappenberger|first5=F.|last6=Barth|first6=J.V.}}</ref>]] |
[[File:MD rotor 250K 1ns.gif|thumb|Molecular dynamics simulation of a synthetic molecular rotor composed of three molecules in a nanopore (outer diameter 6.7 nm) at 250 K.<ref>{{cite journal|title=Topological Dynamics in Supramolecular Rotors|journal=Nano Letters|volume=148|pages=4461–4468|year=2014|last1=Palma|first1=C.-A.|last2=Björk|first2=J.|last3=Rao|first3=F.|last4=Kühne|first4=D.|last5=Klappenberger|first5=F.|last6=Barth|first6=J.V.|issue=8|doi=10.1021/nl5014162|pmid=25078022}}</ref>]] |
||
The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation.{{cn|date=June 2019}} The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of [[Boston College]] with co-workers and the light-driven motor by [[Ben Feringa]] and co-workers, were published in 1999 in the same issue of [[nature (journal)|Nature]]. |
The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation.{{cn|date=June 2019}} The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of [[Boston College]] with co-workers and the light-driven motor by [[Ben Feringa]] and co-workers, were published in 1999 in the same issue of [[nature (journal)|Nature]]. |
||
As of 2020 the smallest, atomically precise molecular machine has a rotor, which consist of four atoms.<ref>{{Cite journal| |
As of 2020 the smallest, atomically precise molecular machine has a rotor, which consist of four atoms.<ref>{{Cite journal|last1=Stolz|first1=Samuel|last2=Gröning|first2=Oliver|last3=Prinz|first3=Jan|last4=Brune|first4=Harald|last5=Widmer|first5=Roland|date=2020-06-15|title=Molecular motor crossing the frontier of classical to quantum tunneling motion|journal=Proceedings of the National Academy of Sciences|volume=117|issue=26|pages=14838–14842|language=en|doi=10.1073/pnas.1918654117|issn=0027-8424|pmid=32541061|pmc=7334648}}</ref> |
||
==Chemically driven rotary molecular motors== |
==Chemically driven rotary molecular motors== |
||
[[Image:Kelly chem motor.png|500px|right|thumb|The prototype of a chemically driven rotary molecular motor by Kelly and co-workers.]]An example of a prototype for a synthetic chemically driven rotary molecular motor was reported by Kelly and co-workers in 1999.<ref>{{cite journal|title=Unidirectional rotary motion in a molecular system|journal=Nature|volume=401|issue=6749|pages=150–2|doi=10.1038/43639|pmid=10490021|year=1999|last1=Kelly|first1=T. R.|last2=De Silva|first2=H|last3=Silva|first3=R. A.|bibcode=1999Natur.401..150K}}</ref> Their system is made up from a three-bladed [[triptycene]] rotor and a [[helicene]], and is capable of performing a unidirectional 120° rotation. |
[[Image:Kelly chem motor.png|500px|right|thumb|The prototype of a chemically driven rotary molecular motor by Kelly and co-workers.]]An example of a prototype for a synthetic chemically driven rotary molecular motor was reported by Kelly and co-workers in 1999.<ref>{{cite journal|title=Unidirectional rotary motion in a molecular system|journal=Nature|volume=401|issue=6749|pages=150–2|doi=10.1038/43639|pmid=10490021|year=1999|last1=Kelly|first1=T. R.|last2=De Silva|first2=H|last3=Silva|first3=R. A.|bibcode=1999Natur.401..150K|s2cid=4351615}}</ref> Their system is made up from a three-bladed [[triptycene]] rotor and a [[helicene]], and is capable of performing a unidirectional 120° rotation. |
||
This rotation takes place in five steps. The [[amine]] group present on the triptycene moiety is converted to an [[isocyanate]] group by condensation with [[phosgene]] ('''a'''). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the [[hydroxyl]] group located on the helicene moiety ('''b'''), thereby allowing these two groups to react with each other ('''c'''). This reaction [[Irreversibility|irreversibly]] traps the system as a [[Strain (chemistry)|strained]] cyclic [[Carbamate|urethane]] that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of [[Activation energy|thermal activation]] in order to overcome this barrier, thereby releasing the strain ('''d'''). Finally, cleavage of the urethane group restores the amine and alcohol [[Functional group|functionalities]] of the molecule ('''e'''). |
This rotation takes place in five steps. The [[amine]] group present on the triptycene moiety is converted to an [[isocyanate]] group by condensation with [[phosgene]] ('''a'''). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the [[hydroxyl]] group located on the helicene moiety ('''b'''), thereby allowing these two groups to react with each other ('''c'''). This reaction [[Irreversibility|irreversibly]] traps the system as a [[Strain (chemistry)|strained]] cyclic [[Carbamate|urethane]] that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of [[Activation energy|thermal activation]] in order to overcome this barrier, thereby releasing the strain ('''d'''). Finally, cleavage of the urethane group restores the amine and alcohol [[Functional group|functionalities]] of the molecule ('''e'''). |
||
| Line 15: | Line 15: | ||
The motor by Kelly and co-workers is an elegant example of how [[chemical energy]] can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of [[Adenosine triphosphate|ATP]] in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned.<ref>{{cite journal|title=Progress toward a Rationally Designed, Chemically Powered Rotary Molecular Motor|journal=Journal of the American Chemical Society|volume=129|issue=2|pages=376–86|doi=10.1021/ja066044a|pmid=17212418|year=2007|last1=Kelly|first1=T. Ross|last2=Cai|first2=Xiaolu|last3=Damkaci|first3=Fehmi|last4=Panicker|first4=Sreeletha B.|last5=Tu|first5=Bin|last6=Bushell|first6=Simon M.|last7=Cornella|first7=Ivan|last8=Piggott|first8=Matthew J.|last9=Salives|first9=Richard|last10=Cavero|first10=Marta|last11=Zhao|first11=Yajun|last12=Jasmin|first12=Serge}}</ref> In 2016 [[David Leigh (scientist)|David Leigh]]'s group invented the first autonomous chemically-fuelled synthetic molecular motor.<ref>{{Cite journal|last1=Wilson|first1=M. R.|last2=Solá|first2=J.|last3=Carlone|first3=A.|last4=Goldup|first4=S. M.|last5=Lebrasseur|first5=N.|last6=Leigh|first6=D. A.|year=2016|title=An autonomous chemically fuelled small-molecule motor|url=https://www.research.manchester.ac.uk/portal/en/publications/an-autonomous-chemically-fuelled-smallmolecule-motor(096f6399-63a0-491a-811b-3b83a543584c).html|journal=Nature|volume=534|issue=7606|pages=235–240|doi=10.1038/nature18013|pmid=27279219|archive-url=http://man.ac.uk/O06viC|archive-date=9 June 2016|bibcode=2016Natur.534..235W}}</ref> |
The motor by Kelly and co-workers is an elegant example of how [[chemical energy]] can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of [[Adenosine triphosphate|ATP]] in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned.<ref>{{cite journal|title=Progress toward a Rationally Designed, Chemically Powered Rotary Molecular Motor|journal=Journal of the American Chemical Society|volume=129|issue=2|pages=376–86|doi=10.1021/ja066044a|pmid=17212418|year=2007|last1=Kelly|first1=T. Ross|last2=Cai|first2=Xiaolu|last3=Damkaci|first3=Fehmi|last4=Panicker|first4=Sreeletha B.|last5=Tu|first5=Bin|last6=Bushell|first6=Simon M.|last7=Cornella|first7=Ivan|last8=Piggott|first8=Matthew J.|last9=Salives|first9=Richard|last10=Cavero|first10=Marta|last11=Zhao|first11=Yajun|last12=Jasmin|first12=Serge}}</ref> In 2016 [[David Leigh (scientist)|David Leigh]]'s group invented the first autonomous chemically-fuelled synthetic molecular motor.<ref>{{Cite journal|last1=Wilson|first1=M. R.|last2=Solá|first2=J.|last3=Carlone|first3=A.|last4=Goldup|first4=S. M.|last5=Lebrasseur|first5=N.|last6=Leigh|first6=D. A.|year=2016|title=An autonomous chemically fuelled small-molecule motor|url=https://www.research.manchester.ac.uk/portal/en/publications/an-autonomous-chemically-fuelled-smallmolecule-motor(096f6399-63a0-491a-811b-3b83a543584c).html|journal=Nature|volume=534|issue=7606|pages=235–240|doi=10.1038/nature18013|pmid=27279219|archive-url=http://man.ac.uk/O06viC|archive-date=9 June 2016|bibcode=2016Natur.534..235W}}</ref> |
||
Some other examples of synthetic chemically driven rotary molecular motors that all operate by sequential addition of reagents have been reported, including the use of the [[Stereoselectivity|stereoselective]] [[Ring-opening reaction|ring opening]] of a [[Racemic mixture|racemic]] [[biaryl]] [[lactone]] by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation.<ref>{{cite journal|title=Net directed 180° aryl–aryl bond rotation in a prototypical achiral biaryl lactone synthetic molecular motor|journal=Tetrahedron Letters|volume=46|issue=48|pages=8359|doi=10.1016/j.tetlet.2005.09.151|year=2005|last1=Lin|first1=Ying|last2=Dahl|first2=Bart J.|last3=Branchaud|first3=Bruce P.}}</ref> Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation.<ref>{{cite journal | doi = 10.1126/science.1117090 | title = A Reversible, Unidirectional Molecular Rotary Motor Driven by Chemical Energy | year = 2005 | last1 = Fletcher | first1 = S. P. | journal = Science | volume = 310 | issue = 5745 | pages = 80–2 | pmid = 16210531 | last2 = Dumur | first2 = F | last3 = Pollard | first3 = MM | last4 = Feringa | first4 = BL | bibcode = 2005Sci...310...80F | url = https://www.rug.nl/research/portal/en/publications/a-reversible-unidirectional-molecular-rotary-motor-driven-by-chemical-energy(50a4c59b-e2fd-413b-a58f-bd37494432e9).html }}</ref> The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the [[aryl]] [[wiktionary:moiety|moiety]] is restricted, although [[Magnetic helicity|helix]] inversion is possible. In stages B and D the aryl can rotate with respect to the [[naphthalene]] with [[steric effects|steric interactions]] preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the [[protecting group|deprotection]] of the [[phenol]], followed by [[Regioselectivity|regioselective]] ring formation. |
Some other examples of synthetic chemically driven rotary molecular motors that all operate by sequential addition of reagents have been reported, including the use of the [[Stereoselectivity|stereoselective]] [[Ring-opening reaction|ring opening]] of a [[Racemic mixture|racemic]] [[biaryl]] [[lactone]] by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation.<ref>{{cite journal|title=Net directed 180° aryl–aryl bond rotation in a prototypical achiral biaryl lactone synthetic molecular motor|journal=Tetrahedron Letters|volume=46|issue=48|pages=8359|doi=10.1016/j.tetlet.2005.09.151|year=2005|last1=Lin|first1=Ying|last2=Dahl|first2=Bart J.|last3=Branchaud|first3=Bruce P.}}</ref> Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation.<ref>{{cite journal | doi = 10.1126/science.1117090 | title = A Reversible, Unidirectional Molecular Rotary Motor Driven by Chemical Energy | year = 2005 | last1 = Fletcher | first1 = S. P. | journal = Science | volume = 310 | issue = 5745 | pages = 80–2 | pmid = 16210531 | last2 = Dumur | first2 = F | last3 = Pollard | first3 = MM | last4 = Feringa | first4 = BL | bibcode = 2005Sci...310...80F | s2cid = 28174183 | url = https://www.rug.nl/research/portal/en/publications/a-reversible-unidirectional-molecular-rotary-motor-driven-by-chemical-energy(50a4c59b-e2fd-413b-a58f-bd37494432e9).html }}</ref> The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the [[aryl]] [[wiktionary:moiety|moiety]] is restricted, although [[Magnetic helicity|helix]] inversion is possible. In stages B and D the aryl can rotate with respect to the [[naphthalene]] with [[steric effects|steric interactions]] preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the [[protecting group|deprotection]] of the [[phenol]], followed by [[Regioselectivity|regioselective]] ring formation. |
||
[[Image:Feringa chem motor.png|650px|center|thumb|The chemically driven rotary molecular motor by Feringa and co-workers.]] |
[[Image:Feringa chem motor.png|650px|center|thumb|The chemically driven rotary molecular motor by Feringa and co-workers.]] |
||
| Line 21: | Line 21: | ||
==Light-driven rotary molecular motors== |
==Light-driven rotary molecular motors== |
||
[[Image:First gen mol motor feringa.png|400px|right|thumb|Rotary cycle of the light-driven rotary molecular motor by Feringa and co-workers.]] |
[[Image:First gen mol motor feringa.png|400px|right|thumb|Rotary cycle of the light-driven rotary molecular motor by Feringa and co-workers.]] |
||
In 1999 the laboratory of [[Ben Feringa|Prof. Dr. Ben L. Feringa]] at the [[University of Groningen]], [[Netherlands|The Netherlands]], reported the creation of a unidirectional molecular rotor.<ref>{{cite journal|title=Light-driven monodirectional molecular rotor|journal=Nature|volume=401|issue=6749|pages=152–5|doi=10.1038/43646|pmid=10490022|year=1999|last1=Feringa|first1=Ben L.|last2=Koumura|first2=Nagatoshi|last3=Zijlstra|first3=Robert W. J.|last4=Van Delden|first4=Richard A.|last5=Harada|first5=Nobuyuki|bibcode=1999Natur.401..152K}}</ref> Their 360° molecular motor system consists of a bis-[[helicene]] connected by an [[alkene]] double bond displaying [[axial chirality]] and having two [[stereocenter]]s. |
In 1999 the laboratory of [[Ben Feringa|Prof. Dr. Ben L. Feringa]] at the [[University of Groningen]], [[Netherlands|The Netherlands]], reported the creation of a unidirectional molecular rotor.<ref>{{cite journal|title=Light-driven monodirectional molecular rotor|journal=Nature|volume=401|issue=6749|pages=152–5|doi=10.1038/43646|pmid=10490022|year=1999|last1=Feringa|first1=Ben L.|last2=Koumura|first2=Nagatoshi|last3=Zijlstra|first3=Robert W. J.|last4=Van Delden|first4=Richard A.|last5=Harada|first5=Nobuyuki|bibcode=1999Natur.401..152K|s2cid=4412610|url=https://pure.rug.nl/ws/files/3616669/1999NatureKoumura.pdf}}</ref> Their 360° molecular motor system consists of a bis-[[helicene]] connected by an [[alkene]] double bond displaying [[axial chirality]] and having two [[stereocenter]]s. |
||
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature [[endothermic reaction|endothermic]] [[photoisomerization]] of the [[trans isomer|trans]] (''P'',''P'') isomer '''1''' to the [[cis isomer|''cis'']] (''M'',''M'') '''2''' where ''P'' stands for the right-handed [[helix]] and ''M'' for the left-handed helix. In this process, the two [[axial bond|axial]] [[methyl]] groups are converted into two less [[steric hindrance|sterically]] favorable [[equatorial bond|equatorial]] methyl groups. |
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature [[endothermic reaction|endothermic]] [[photoisomerization]] of the [[trans isomer|trans]] (''P'',''P'') isomer '''1''' to the [[cis isomer|''cis'']] (''M'',''M'') '''2''' where ''P'' stands for the right-handed [[helix]] and ''M'' for the left-handed helix. In this process, the two [[axial bond|axial]] [[methyl]] groups are converted into two less [[steric hindrance|sterically]] favorable [[equatorial bond|equatorial]] methyl groups. |
||
| Line 30: | Line 30: | ||
A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a [[fluorene]] lower half, the half-life of the thermal helix inversion is 0.005 seconds.<ref>{{cite journal|title=Fine Tuning of the Rotary Motion by Structural Modification in Light-Driven Unidirectional Molecular Motors|journal=Journal of the American Chemical Society|volume=128|issue=15|pages=5127–35|doi=10.1021/ja058303m|pmid=16608348|year=2006|last1=Vicario|first1=Javier|last2=Walko|first2=Martin|last3=Meetsma|first3=Auke|last4=Feringa|first4=Ben L.|url=https://pure.rug.nl/ws/files/10181731/2006JAmChemSocVicario.pdf}}</ref> This compound is synthesized using the [[Barton-Kellogg reaction]]. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger [[tert-butyl|''tert''-butyl]] group makes the unstable isomer even less stable than when the [[methyl]] group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a ''tert''-butyl group is 3.2 minutes.<ref>{{cite journal|title=Controlling the speed of rotation in molecular motors. Dramatic acceleration of the rotary motion by structural modification|journal=Chemical Communications|issue=47|pages=5910–2|doi=10.1039/b507264f|pmid=16317472|year=2005|last1=Vicario|first1=Javier|last2=Meetsma|first2=Auke|last3=Feringa|first3=Ben L.}}</ref> |
A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a [[fluorene]] lower half, the half-life of the thermal helix inversion is 0.005 seconds.<ref>{{cite journal|title=Fine Tuning of the Rotary Motion by Structural Modification in Light-Driven Unidirectional Molecular Motors|journal=Journal of the American Chemical Society|volume=128|issue=15|pages=5127–35|doi=10.1021/ja058303m|pmid=16608348|year=2006|last1=Vicario|first1=Javier|last2=Walko|first2=Martin|last3=Meetsma|first3=Auke|last4=Feringa|first4=Ben L.|url=https://pure.rug.nl/ws/files/10181731/2006JAmChemSocVicario.pdf}}</ref> This compound is synthesized using the [[Barton-Kellogg reaction]]. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger [[tert-butyl|''tert''-butyl]] group makes the unstable isomer even less stable than when the [[methyl]] group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a ''tert''-butyl group is 3.2 minutes.<ref>{{cite journal|title=Controlling the speed of rotation in molecular motors. Dramatic acceleration of the rotary motion by structural modification|journal=Chemical Communications|issue=47|pages=5910–2|doi=10.1039/b507264f|pmid=16317472|year=2005|last1=Vicario|first1=Javier|last2=Meetsma|first2=Auke|last3=Feringa|first3=Ben L.}}</ref> |
||
The Feringa principle has been incorporated into a prototype [[nanocar]].<ref>{{cite journal|title=En Route to a Motorized Nanocar|journal=Organic Letters |volume=8 |issue=8 |pages=1713–6 |doi=10.1021/ol060445d |pmid=16597148 |year=2006 |last1=Morin |first1=Jean-François |last2=Shirai |first2=Yasuhiro |last3=Tour |first3=James M. }}</ref> The car [[organic synthesis|synthesized]] has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four [[carborane]] wheels and is expected to be able to move on a solid surface with [[scanning tunneling microscopy]] monitoring, although so far this has not been observed. The motor does not perform with [[fullerene]] wheels because they [[quenching|quench]] the photochemistry of the motor [[Moiety (chemistry)|moiety]]. Feringa motors have also been shown to remain operable when chemically attached to solid surfaces.<ref>{{cite journal|title=Controlled rotary motion of light-driven molecular motors assembled on a gold film|journal=Chemical Science|volume=1|pages=97|doi=10.1039/C0SC00162G|year=2010|last1=Carroll|first1=Gregory T.|last2=Pollard|first2=Michael M.|last3=Van Delden|first3=Richard|last4=Feringa|first4=Ben L.|url=https://www.rug.nl/research/portal/en/publications/controlled-rotary-motion-of-lightdriven-molecular-motors-assembled-on-a-gold-film(4fb63d6d-d764-45e3-b3cb-32a4c629b942).html}}</ref><ref>{{cite journal|title=Adhesion of photon-driven molecular motors to surfaces via 1, 3-dipolar cycloadditions: Effect of interfacial interactions on molecular motion|journal=ACS Nano|volume=5|issue=1|pages=622–30|doi=10.1021/nn102876j|pmid=21207983|year=2011|last1=Carroll|first1=Gregory T.|last2=London|first2=Gábor|last3=Landaluce|first3=Tatiana FernáNdez|last4=Rudolf|first4=Petra|authorlink4=Petra Rudolf|last5=Feringa|first5=Ben L.|url=https://pure.rug.nl/ws/files/6757528/2011ACSNanoCarroll.pdf}}</ref> The ability of certain Feringa systems to act as an [[asymmetric catalyst]] has also been demonstrated.<ref>{{cite journal|title=Dynamic Control of Chiral Space in a Catalytic Asymmetric Reaction Using a Molecular Motor Science|journal=Science|volume=331|issue=6023|pages=1429–32|doi=10.1126/science.1199844|pmid=21310964|year=2011|last1=Wang|first1=J.|last2=Feringa|first2=B. L.|bibcode=2011Sci...331.1429W}}</ref><ref>{{cite journal|title=Heat and Light Switch a Chiral Catalyst and Its Products|journal=Science|volume=331|issue=6023|pages=1395–6|doi=10.1126/science.1203272|pmid=21415343|year=2011|last1=Ooi|first1=T.|bibcode=2011Sci...331.1395O}}</ref> |
The Feringa principle has been incorporated into a prototype [[nanocar]].<ref>{{cite journal|title=En Route to a Motorized Nanocar|journal=Organic Letters |volume=8 |issue=8 |pages=1713–6 |doi=10.1021/ol060445d |pmid=16597148 |year=2006 |last1=Morin |first1=Jean-François |last2=Shirai |first2=Yasuhiro |last3=Tour |first3=James M. }}</ref> The car [[organic synthesis|synthesized]] has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four [[carborane]] wheels and is expected to be able to move on a solid surface with [[scanning tunneling microscopy]] monitoring, although so far this has not been observed. The motor does not perform with [[fullerene]] wheels because they [[quenching|quench]] the photochemistry of the motor [[Moiety (chemistry)|moiety]]. Feringa motors have also been shown to remain operable when chemically attached to solid surfaces.<ref>{{cite journal|title=Controlled rotary motion of light-driven molecular motors assembled on a gold film|journal=Chemical Science|volume=1|pages=97|doi=10.1039/C0SC00162G|year=2010|last1=Carroll|first1=Gregory T.|last2=Pollard|first2=Michael M.|last3=Van Delden|first3=Richard|last4=Feringa|first4=Ben L.|url=https://www.rug.nl/research/portal/en/publications/controlled-rotary-motion-of-lightdriven-molecular-motors-assembled-on-a-gold-film(4fb63d6d-d764-45e3-b3cb-32a4c629b942).html}}</ref><ref>{{cite journal|title=Adhesion of photon-driven molecular motors to surfaces via 1, 3-dipolar cycloadditions: Effect of interfacial interactions on molecular motion|journal=ACS Nano|volume=5|issue=1|pages=622–30|doi=10.1021/nn102876j|pmid=21207983|year=2011|last1=Carroll|first1=Gregory T.|last2=London|first2=Gábor|last3=Landaluce|first3=Tatiana FernáNdez|last4=Rudolf|first4=Petra|authorlink4=Petra Rudolf|last5=Feringa|first5=Ben L.|url=https://pure.rug.nl/ws/files/6757528/2011ACSNanoCarroll.pdf}}</ref> The ability of certain Feringa systems to act as an [[asymmetric catalyst]] has also been demonstrated.<ref>{{cite journal|title=Dynamic Control of Chiral Space in a Catalytic Asymmetric Reaction Using a Molecular Motor Science|journal=Science|volume=331|issue=6023|pages=1429–32|doi=10.1126/science.1199844|pmid=21310964|year=2011|last1=Wang|first1=J.|last2=Feringa|first2=B. L.|bibcode=2011Sci...331.1429W|s2cid=24556473}}</ref><ref>{{cite journal|title=Heat and Light Switch a Chiral Catalyst and Its Products|journal=Science|volume=331|issue=6023|pages=1395–6|doi=10.1126/science.1203272|pmid=21415343|year=2011|last1=Ooi|first1=T.|bibcode=2011Sci...331.1395O|s2cid=206532839}}</ref> |
||
In 2016, Feringa was awarded a Nobel prize for his work on molecular motors. |
In 2016, Feringa was awarded a Nobel prize for his work on molecular motors. |
||
Revision as of 08:01, 11 September 2020
Synthetic molecular motors are molecular machines capable of continuous directional rotation under an energy input.[1] Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via protein dynamics), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors.

The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation.[citation needed] The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of Boston College with co-workers and the light-driven motor by Ben Feringa and co-workers, were published in 1999 in the same issue of Nature.
As of 2020 the smallest, atomically precise molecular machine has a rotor, which consist of four atoms.[3]
Chemically driven rotary molecular motors
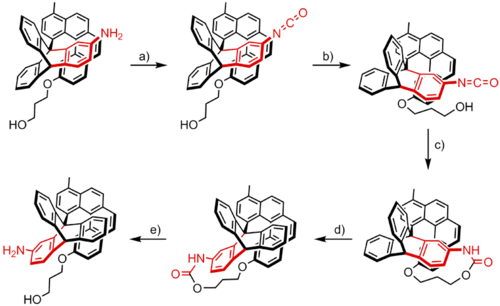
An example of a prototype for a synthetic chemically driven rotary molecular motor was reported by Kelly and co-workers in 1999.[4] Their system is made up from a three-bladed triptycene rotor and a helicene, and is capable of performing a unidirectional 120° rotation.
This rotation takes place in five steps. The amine group present on the triptycene moiety is converted to an isocyanate group by condensation with phosgene (a). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the hydroxyl group located on the helicene moiety (b), thereby allowing these two groups to react with each other (c). This reaction irreversibly traps the system as a strained cyclic urethane that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of thermal activation in order to overcome this barrier, thereby releasing the strain (d). Finally, cleavage of the urethane group restores the amine and alcohol functionalities of the molecule (e).
The result of this sequence of events is a unidirectional 120° rotation of the triptycene moiety with respect to the helicene moiety. Additional forward or backward rotation of the triptycene rotor is inhibited by the helicene moiety, which serves a function similar to that of the pawl of a ratchet. The unidirectionality of the system is a result from both the asymmetric skew of the helicene moiety as well as the strain of the cyclic urethane which is formed in c. This strain can be only be lowered by the clockwise rotation of the triptycene rotor in d, as both counterclockwise rotation as well as the inverse process of d are energetically unfavorable. In this respect the preference for the rotation direction is determined by both the positions of the functional groups and the shape of the helicene and is thus built into the design of the molecule instead of dictated by external factors.
The motor by Kelly and co-workers is an elegant example of how chemical energy can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of ATP in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned.[5] In 2016 David Leigh's group invented the first autonomous chemically-fuelled synthetic molecular motor.[6]
Some other examples of synthetic chemically driven rotary molecular motors that all operate by sequential addition of reagents have been reported, including the use of the stereoselective ring opening of a racemic biaryl lactone by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation.[7] Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation.[8] The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the aryl moiety is restricted, although helix inversion is possible. In stages B and D the aryl can rotate with respect to the naphthalene with steric interactions preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the deprotection of the phenol, followed by regioselective ring formation.
Light-driven rotary molecular motors

In 1999 the laboratory of Prof. Dr. Ben L. Feringa at the University of Groningen, The Netherlands, reported the creation of a unidirectional molecular rotor.[9] Their 360° molecular motor system consists of a bis-helicene connected by an alkene double bond displaying axial chirality and having two stereocenters.
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature endothermic photoisomerization of the trans (P,P) isomer 1 to the cis (M,M) 2 where P stands for the right-handed helix and M for the left-handed helix. In this process, the two axial methyl groups are converted into two less sterically favorable equatorial methyl groups.
By increasing the temperature to 20 °C these methyl groups convert back exothermally to the (P,P) cis axial groups (3) in a helix inversion. Because the axial isomer is more stable than the equatorial isomer, reverse rotation is blocked. A second photoisomerization converts (P,P) cis 3 into (M,M) trans 4, again with accompanying formation of sterically unfavorable equatorial methyl groups. A thermal isomerization process at 60 °C closes the 360° cycle back to the axial positions.

A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a fluorene lower half, the half-life of the thermal helix inversion is 0.005 seconds.[10] This compound is synthesized using the Barton-Kellogg reaction. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger tert-butyl group makes the unstable isomer even less stable than when the methyl group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a tert-butyl group is 3.2 minutes.[11]
The Feringa principle has been incorporated into a prototype nanocar.[12] The car synthesized has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four carborane wheels and is expected to be able to move on a solid surface with scanning tunneling microscopy monitoring, although so far this has not been observed. The motor does not perform with fullerene wheels because they quench the photochemistry of the motor moiety. Feringa motors have also been shown to remain operable when chemically attached to solid surfaces.[13][14] The ability of certain Feringa systems to act as an asymmetric catalyst has also been demonstrated.[15][16]
In 2016, Feringa was awarded a Nobel prize for his work on molecular motors.
Experimental demonstration of a single-molecule electric motor
A single-molecule electrically operated motor made from a single molecule of n-butyl methyl sulfide (C5H12S) has been reported. The molecule is adsorbed onto a copper (111) single-crystal piece by chemisorption.[citation needed]
See also
References
- ^ Kassem, Salma; van Leeuwen, Thomas; Lubbe, Anouk S.; Wilson, Miriam R.; Feringa, Ben L.; Leigh, David A. (2017). "Artificial molecular motors". Chemical Society Reviews. 46 (9): 2592–2621. doi:10.1039/C7CS00245A. PMID 28426052.
- ^ Palma, C.-A.; Björk, J.; Rao, F.; Kühne, D.; Klappenberger, F.; Barth, J.V. (2014). "Topological Dynamics in Supramolecular Rotors". Nano Letters. 148 (8): 4461–4468. doi:10.1021/nl5014162. PMID 25078022.
- ^ Stolz, Samuel; Gröning, Oliver; Prinz, Jan; Brune, Harald; Widmer, Roland (2020-06-15). "Molecular motor crossing the frontier of classical to quantum tunneling motion". Proceedings of the National Academy of Sciences. 117 (26): 14838–14842. doi:10.1073/pnas.1918654117. ISSN 0027-8424. PMC 7334648. PMID 32541061.
- ^ Kelly, T. R.; De Silva, H; Silva, R. A. (1999). "Unidirectional rotary motion in a molecular system". Nature. 401 (6749): 150–2. Bibcode:1999Natur.401..150K. doi:10.1038/43639. PMID 10490021. S2CID 4351615.
- ^ Kelly, T. Ross; Cai, Xiaolu; Damkaci, Fehmi; Panicker, Sreeletha B.; Tu, Bin; Bushell, Simon M.; Cornella, Ivan; Piggott, Matthew J.; Salives, Richard; Cavero, Marta; Zhao, Yajun; Jasmin, Serge (2007). "Progress toward a Rationally Designed, Chemically Powered Rotary Molecular Motor". Journal of the American Chemical Society. 129 (2): 376–86. doi:10.1021/ja066044a. PMID 17212418.
- ^ Wilson, M. R.; Solá, J.; Carlone, A.; Goldup, S. M.; Lebrasseur, N.; Leigh, D. A. (2016). "An autonomous chemically fuelled small-molecule motor". Nature. 534 (7606): 235–240. Bibcode:2016Natur.534..235W. doi:10.1038/nature18013. PMID 27279219. Archived from the original on 9 June 2016.
- ^ Lin, Ying; Dahl, Bart J.; Branchaud, Bruce P. (2005). "Net directed 180° aryl–aryl bond rotation in a prototypical achiral biaryl lactone synthetic molecular motor". Tetrahedron Letters. 46 (48): 8359. doi:10.1016/j.tetlet.2005.09.151.
- ^ Fletcher, S. P.; Dumur, F; Pollard, MM; Feringa, BL (2005). "A Reversible, Unidirectional Molecular Rotary Motor Driven by Chemical Energy". Science. 310 (5745): 80–2. Bibcode:2005Sci...310...80F. doi:10.1126/science.1117090. PMID 16210531. S2CID 28174183.
- ^ Feringa, Ben L.; Koumura, Nagatoshi; Zijlstra, Robert W. J.; Van Delden, Richard A.; Harada, Nobuyuki (1999). "Light-driven monodirectional molecular rotor" (PDF). Nature. 401 (6749): 152–5. Bibcode:1999Natur.401..152K. doi:10.1038/43646. PMID 10490022. S2CID 4412610.
- ^ Vicario, Javier; Walko, Martin; Meetsma, Auke; Feringa, Ben L. (2006). "Fine Tuning of the Rotary Motion by Structural Modification in Light-Driven Unidirectional Molecular Motors" (PDF). Journal of the American Chemical Society. 128 (15): 5127–35. doi:10.1021/ja058303m. PMID 16608348.
- ^ Vicario, Javier; Meetsma, Auke; Feringa, Ben L. (2005). "Controlling the speed of rotation in molecular motors. Dramatic acceleration of the rotary motion by structural modification". Chemical Communications (47): 5910–2. doi:10.1039/b507264f. PMID 16317472.
- ^ Morin, Jean-François; Shirai, Yasuhiro; Tour, James M. (2006). "En Route to a Motorized Nanocar". Organic Letters. 8 (8): 1713–6. doi:10.1021/ol060445d. PMID 16597148.
- ^ Carroll, Gregory T.; Pollard, Michael M.; Van Delden, Richard; Feringa, Ben L. (2010). "Controlled rotary motion of light-driven molecular motors assembled on a gold film". Chemical Science. 1: 97. doi:10.1039/C0SC00162G.
- ^ Carroll, Gregory T.; London, Gábor; Landaluce, Tatiana FernáNdez; Rudolf, Petra; Feringa, Ben L. (2011). "Adhesion of photon-driven molecular motors to surfaces via 1, 3-dipolar cycloadditions: Effect of interfacial interactions on molecular motion" (PDF). ACS Nano. 5 (1): 622–30. doi:10.1021/nn102876j. PMID 21207983.
- ^ Wang, J.; Feringa, B. L. (2011). "Dynamic Control of Chiral Space in a Catalytic Asymmetric Reaction Using a Molecular Motor Science". Science. 331 (6023): 1429–32. Bibcode:2011Sci...331.1429W. doi:10.1126/science.1199844. PMID 21310964. S2CID 24556473.
- ^ Ooi, T. (2011). "Heat and Light Switch a Chiral Catalyst and Its Products". Science. 331 (6023): 1395–6. Bibcode:2011Sci...331.1395O. doi:10.1126/science.1203272. PMID 21415343. S2CID 206532839.
