Allyl alcohol
| |
| |
| Names | |
|---|---|
| IUPAC name
2-propenol, Allyl alcohol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.156 |
| KEGG | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6O | |
| Molar mass | 58.080 g·mol−1 |
| Density | 0.854 g/ml |
| Melting point | −129 °C |
| Boiling point | 97 °C |
| Miscible | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 21 °C |
| Explosive limits | 2.5–18.0% |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
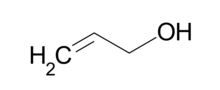
Allyl alcohol (IUPAC name: 2-propenol) is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is also used as a precursor to many specialized compounds. Allyl alcohol is the smallest representative of the allylic alcohols.
Production
Allyl alcohol can be obtained by many methods.[1] The hydrolysis of allyl chloride is the traditional route:
- CH2CHCH2Cl + NaOH → CH2CHCH2OH + NaCl
Allyl alcohol can also be made by the rearrangement of propylene oxide, a reaction that is catalyzed by potassium alum at high temperature. The advantage of this method relative to the allyl chloride route is that it does not generate salt. Also avoiding chloride-containing intermediates is the "acetoxylation" of propylene to allyl acetate:
- CH2=CHCH3 + 1/2 O2 + CH3CO2H → CH2CHCH2O2CCH3 + H2O
Hydrolysis of this acetate gives allyl alcohol. Alternatively, propylene can be oxidized to acrolein, which upon hydrogenation gives the alcohol.
Other methods
In principle allyl alcohol can be obtained by dehydrogenation of propanol. In the laboratory, it has been prepared by the reaction of glycerol and formic acid.[2] Allyl alcohols in general can be prepared by allylic oxidation of allyl compounds by selenium dioxide.
Applications
Allyl alcohol is mainly converted to glycidol, which is a chemical intermediate in the synthesis of glycerol, glycidyl ethers, esters and amines. Also, a variety of polymerizable esters are prepared from allyl alcohol, e.g. diallyl phthalate.[1]
Safety
Allyl alcohol is more toxic than related alcohols. Its threshold limit value (TLV) is 2 ppm. It is a lacrymator.[1]
References
- ^ a b c Ludger Krähling, Jürgen Krey, Gerald Jakobson, Johann Grolig, Leopold Miksche “Allyl Compounds” Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_425
- ^ Oliver Kamm and C. S. Marvel (1941). "Allyl alcohol". Organic Syntheses; Collected Volumes, vol. 1, p. 42.
External links
- International Chemical Safety Card 0095
- NIOSH Pocket Guide to Chemical Hazards. "#0017". National Institute for Occupational Safety and Health (NIOSH).
- Institut national de recherche et de sécurité (2004). "Alcool allylique." Fiche toxicologique n° 156. Paris:INRS. (in French)
- State of Michigan public information on allyl alcohol
- Occupational exposure guidelines
- Datasheet regulatory information

