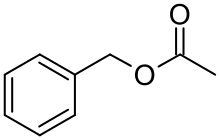
Benzyl acetate
| |

| |
| Names | |
|---|---|
| IUPAC name
Benzyl acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.909 |
| KEGG | |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H5CH2OCOCH3 | |
| Molar mass | 150.18 g/mol |
| Density | 1.054 g/ml |
| Melting point | −51 °C (−60 °F; 222 K) |
| Boiling point | 212 °C (414 °F; 485 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl acetate is an organic compound with the molecular formula C9H10O2. It is the ester formed by condensation of benzyl alcohol and acetic acid.
Benzyl acetate is found naturally in many flowers. It is the primary constituent of the essential oils from the flowers jasmine, ylang-ylang and tobira. It has pleasant sweet aroma reminiscent of jasmine. Consequently, it is used widely in perfumery and cosmetics for its aroma and in flavorings to impart apple and pear flavors.[1]
It is one of many compounds that is attractive to males of various species of orchid bees, who apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.[2]
Benzyl acetate is also used as a solvent in plastics and resin, cellulose acetate, nitrate, oils, lacquers, polishes and inks.[citation needed]
References
- ^ http://www.thegoodscentscompany.com/data/rw1001611.html
- ^ Schiestl, F.P. & Roubik, D.W. (2004). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
