Benzylamine
| |
| Names | |
|---|---|
| IUPAC name
1-Phenylmethanamine
| |
| Other names
Aminotoluene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.595 |
| KEGG | |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H9N | |
| Molar mass | 107.156 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.981 g/mL |
| Melting point | -30 °C (-22°F) |
| Boiling point | 183 °C (361.4°F) |
| 3.24 g / 100 g water @ 25 °C | |
| Solubility in methanol | methanol 9.16 M [1] |
| Acidity (pKa) | 9.34[2] |
| Basicity (pKb) | 4.66 |
Refractive index (nD)
|
1.543 |
| Structure | |
| 1.38 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable |
| NFPA 704 (fire diamond) | |
| Flash point | 72 °C (161.6°F) |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
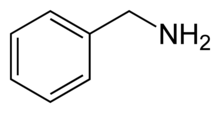
Benzylamine is the chemical compound with the formula C6H5CH2NH2. It consists of a benzyl group, C6H5CH2, attached to an amine functional group. This colorless liquid is a common precursor in organic synthesis.
Benzylamine is preprared by hydrogenation of benzonitrile.
It is used as a masked source of ammonia, since after N-alkylation, the benzyl group can be removed by hydrogenolysis:[3]
- C6H5CH2NH2 + 2 RBr → C6H5CH2NR2 + 2 HBr
- C6H5CH2NR2 + H2 → C6H5CH3 + R2NH
Typically a base is employed in the first step to absorb the HBr (or related acid for other kinds of alkylating agents).
References
- ^ Solubility of benzylamine in methanol
- ^ Hall, H.K., J. Am. Chem. Soc., 1957, 79, 5441.
- ^ Gatto, V. J.; Miller, S. R.; Gokel, G. W. (1993). "4,13-Diaza-18-Crown-6". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 152. (example of alklylation of benzylamine followed by hydrogenolysis).


