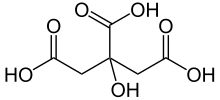
Citric acid
| |

| |
| Names | |
|---|---|
| IUPAC name
2-hydroxypropane-1,2,3-tricarboxylic acid
| |
| Other names
3-carboxy-3-hydroxypentanedioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.973 |
| E number | E330 (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8O7 | |
| Molar mass | 192.124 g/mol (anhydrous) 210.14 g/mol (monohydrate) |
| Appearance | crystalline white solid |
| Density | 1.665 g/cm3(1.5g/cm3 for monohydrate) |
| Melting point | 153 °C (307 °F; 426 K) |
| Boiling point | 175 °C (347 °F; 448 K) |
| 73 g/100 ml (20 °C) | |
| Acidity (pKa) | pKa1 = 3.09 pKa2 = 4.75 pKa3 = 5.41[1] pKa3 = 6.39,[2] 6.40[3] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
skin and eye irritant |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks. In biochemistry, the conjugate base of citric acid, citrate, is important as an intermediate in the citric acid cycle, and therefore occurs in the metabolism of virtually all living things.
Citric acid is a commodity chemical, and more than a million tonnes are produced every year by fermentation. It is used mainly as an acidifier, as a flavoring, and as a chelating agent.
Properties

At room temperature, citric acid is a white crystalline powder. It can exist either in an anhydrous (water-free) form or as a monohydrate. The anhydrous form crystallizes from hot water, whereas the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form by heating above 78 °C. Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C.
In chemical structure, citric acid shares the properties of other carboxylic acids. When heated above 175 °C, it decomposes through the loss of carbon dioxide and water (see decarboxylation).
Citric acid is a slightly stronger acid than typical carboxylic acids because the anion can be stabilized by intramolecular hydrogen-bonding from other protic groups on citric acid.
Discovery and production

The discovery of citric acid has been credited to the 8th century Persian alchemist Jābir ibn Hayyān (Geber).[4] Medieval scholars in Europe were aware of the acidic nature of lemon and lime juices; such knowledge is recorded in the 13th century encyclopedia Speculum Maius (The Great Mirror), compiled by Vincent of Beauvais.[citation needed] Citric acid was first isolated in 1784 by the Swedish chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.[5][6] Industrial-scale citric acid production began in 1890 based on the Italian citrus fruit industry.
In 1893, C. Wehmer discovered Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports. In 1917, the American food chemist James Currie discovered certain strains of the mold Aspergillus niger could be efficient citric acid producers, and the pharmaceutical company Pfizer began industrial-level production using this technique two years later, followed by Citrique Belge in 1929.
In this production technique, which is still the major industrial route to citric acid used today, cultures of A. niger are fed on a sucrose or glucose-containing medium to produce citric acid. The source of sugar is corn steep liquor, molasses, hydrolyzed corn starch or other inexpensive sugary solutions.[7] After the mold is filtered out of the resulting solution, citric acid is isolated by precipitating it with lime (calcium hydroxide) to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid.
Other methods
Prior to the fermentative process, citric acid was isolated from citrus fruits. The juice was treated with lime (Ca(OH)2) to precipitate calcium citrate, which was isolated and converted back to the acid.[5]
In 2007, world wide annual production stood at approximately 1,600,000 tonnes.[8] More than 50% of this volume was produced in China. More than 50% was used as acidulent in beverages, some 20% in other food applications, 20% for detergent applications and 10% for related applications other than food, such as cosmetics, pharmaceutics and in the chemical industry.
Occurrence
Citric acid exists in greater than trace amounts in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices[9]). The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes. Within species, these values vary depending on the cultivar and the circumstances in which the fruit was grown.
Biochemistry
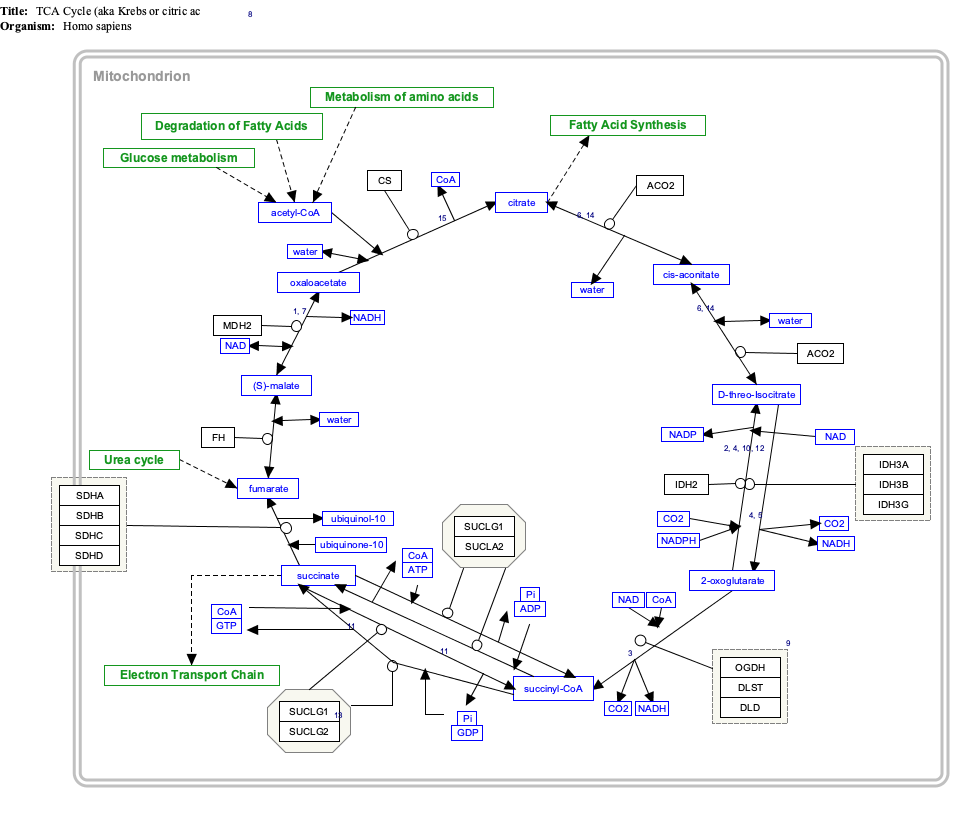
Citric acid cycle
Citrate, the conjugate base of citric acid is one of a series of compounds involved in the physiological oxidation of fats, proteins, and carbohydrates to carbon dioxide and water.
This series of chemical reactions is central to nearly all metabolic reactions, and is the source of two-thirds of the food-derived energy in higher organisms. Hans Adolf Krebs received the 1953 Nobel Prize in Physiology or Medicine for the discovery. The series of reactions is known by various names, including the "citric acid cycle", the "Krebs cycle" or "Szent-Györgyi — Krebs cycle", and the "tricarboxylic acid (TCA) cycle". Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
Other biological roles
Citrate is a critical component of bone, helping to regulate the size of calcium crystals.[10]
Applications
The dominant use of citric acid is as a flavoring and preservative in food and beverages, especially soft drinks.[5] Within the European Union it is denoted by E number E330. Citrate salts of various metals are used to deliver those minerals in a biologically available form in many dietary supplements. The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals. In the United States the purity requirements for citric acid as a food additive are defined by the Food Chemical Codex, which is published by the United States Pharmacopoeia (USP).
Foods, other
Citric acid can be added to e.g. ice cream as an emulsifying agent to keep fats from separating, to caramel to prevent sucrose crystallization, or to recipes in place of fresh lemon juice. Citric acid is used with sodium bicarbonate in a wide range of effervescent formulae, both for ingestion (e.g., powders and tablets) and for personal care (e.g., bath salts, bath bombs, and cleaning of grease). Citric acid is also often used in cleaning products and sodas or fizzy drinks.
Citric acid sold in a dry powdered form is commonly sold in markets and groceries as "sour salt", due to its physical resemblance to table salt. It has use in culinary applications where an acid is needed for either its chemical properties or for its sour flavor, but a dry ingredient is needed and additional flavors are unwanted (e.g., instead of vinegar or lemon juice).
Cleaning and chelating agent
Citric acid is an excellent chelating agent, binding metals. It is used to remove scale from boilers and evaporators.[5] It can be used to soften water, which makes it useful in soaps and laundry detergents. By chelating the metals in hard water, it lets these cleaners produce foam and work better without need for water softening. Citric acid is the active ingredient in some bathroom and kitchen cleaning solutions. A solution with a 6% concentration of citric acid will remove hard water stains from glass without scrubbing. In industry, it is used to dissolve rust from steel. Citric acid can be used in shampoo to wash out wax and coloring from the hair.
Illustrative of its chelating abilities, citric acid was the first successful eluant used for total ion-exchange separation of the lanthanides, during the Manhattan Project in the 1940s. In the 1950s, it was replaced by the far more efficient EDTA. It can be used to slow setting of Portland cement. It can delay setting time substantially.
Cosmetics and pharmaceuticals
Citric acid is widely used as a pH adjusting agent in creams and gels of all kinds. In this role it is classified in most jurisdictions as a processing aid and so does not need to be listed on ingredient lists. Citric acid is an alpha hydroxy acid and used as an active ingredient in chemical peels.
- Citric acid is commonly used as a buffer to increase the solubility of brown heroin. Single-use citric acid sachets have been used as an inducement to get heroin users to exchange their dirty needles for clean needles in an attempt to decrease the spread of AIDS and hepatitis.[11] Other acidifiers used for brown heroin are ascorbic acid, acetic acid, and lactic acid; in their absence, a drug user will often substitute lemon juice or vinegar.
- Citric acid is used as one of the active ingredients in the production of antiviral tissues.[12]
Dyeing
Citric acid can be used in food coloring to balance the pH level of a normally basic dye. It is used as an odorless alternative to white vinegar for home dyeing with acid dyes.
Photography
- Citric acid can be used as a lower-odor stop bath as part of the process for developing photographic film. Photographic developers are alkaline, so a mild acid is used to neutralize and stop their action quickly, but commonly used acetic acid leaves a strong vinegar odor in the darkroom.
Compendial status
See also
- The closely related acids isocitric acid, aconitic acid, and propane-1,2,3-tricarboxylic acid (tricarballylic acid, carballylic acid)
- Acids in wine
Notes
- ^ Dawson, R. M. C.; et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
{{cite book}}: Explicit use of et al. in:|last2=(help) - ^ "Data for Biochemical Research". ZirChrom Separations, Inc. Retrieved January 11, 2012.
- ^ "Ionization Constants of Organic Acids". Michigan State University. Retrieved January 11, 2012.
- ^ Derewenda, Zygmunt S. (2007). "On wine, chirality and crystallography". Acta Crystallographica Section A. 64 (1): 246–258. doi:10.1107/S0108767307054293. ISSN 0108-7673. PMID 18156689.
- ^ a b c d Frank H. Verhoff. "Citric Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. ISBN 978-3527306732.
- ^ Graham, Thomas (1842). Elements of chemistry, including the applications of the science in the arts. Hippolyte Baillière, foreign bookseller to the Royal College of Surgeons, and to the Royal Society, 219, Regent Street. p. 944. Retrieved June 4, 2010.
- ^ Lotfy, Walid A.; Ghanem, Khaled M.; El-Helow, Ehab R. (2007). "Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs". Bioresource Technology. 98 (18): 3470–3477. doi:10.1016/j.biortech.2006.11.032.
- ^ "Citric Acid Production". PMID 17875481.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Penniston KL, Nakada SY, Holmes RP, Assimos DG (2008). "Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products" (PDF). Journal of Endourology. 22 (3): 567–70. doi:10.1089/end.2007.0304. PMC 2637791. PMID 18290732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hu, Y.- Y.; Rawal, A.; Schmidt-Rohr, K. (2010). "Strongly bound citrate stabilizes the apatite nanocrystals in bone". Proceedings of the National Academy of Sciences. 107: 22425. doi:10.1073/pnas.1009219107.
- ^ Garden, J., Roberts, K., Taylor, A., and Robinson, D. (2003). "Evaluation of the Provision of Single Use Citric Acid Sachets to Injecting Drug Users" (pdf). Scottish Center for Infection and Environmental Health.
- ^ "Tissues that fight germs". CNN. July 14, 2004. Retrieved May 8, 2008.
- ^ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Retrieved February 4, 2010.
- ^ "Japanese Pharmacopoeia, Fifteenth Edition" (PDF). 2006. Retrieved 4 Februally 2010.
{{cite web}}: Check date values in:|accessdate=(help)
External links
- CID 311 from PubChem
- The European Citric Acid Manufacturers Association
- NextBio Citric Acid Entry
- Citric acid analysis – free spreadsheet for titration of acids and pH calculation
- Applications of Citric Acid
- MSDS sheet for Citric Acid