Dental implant: Difference between revisions
→External links: added a link to a scholarly publication on dental implants |
|||
| Line 162: | Line 162: | ||
'''Publications''' |
'''Publications''' |
||
*[http://www.implantoloji.info/ Journal of Dental Implantology] |
*[http://www.implantoloji.info/ Journal of Dental Implantology] |
||
*[http://www.ahcpub.com/products_and_services/?prid=303/ Dental Implantology Update] |
|||
Revision as of 14:35, 19 March 2009
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (February 2008) |

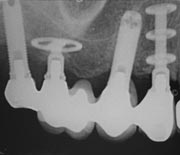
A dental implant is an artificial tooth root replacement and is used in prosthetic dentistry to support restorations that resemble a tooth or group of teeth. There are several types of dental implants. The major classifications are divided into osseointegrated implant and the fibrointegrated implant. Earlier implants, such as the subperiosteal implant and the blade implant were usually fibrointegrated [1], [2]. The most widely accepted and successful implant today is the osseointegrated implant, based on the discovery by Swedish Professor Per-Ingvar Brånemark that titanium can be successfully fused into bone when osteoblasts grow on and into the rough surface of the implanted titanium [3]. This forms a structural and functional connection between the living bone and the implant. A variation on the implant procedure is the implant-supported bridge, or implant-supported denture.
History
The Mayan civilization has been shown to have used the earliest known examples of endosseous implants (implants embedded into bone), dating back over 1,350 years before Per-Ingvar Brånemark started working with titanium. While excavating Mayan burial sites in Honduras in 1931, archaeologists found a fragment of mandible of Mayan origin, dating from about 600 AD. This mandible, which is considered to be that of a woman in her twenties, had three tooth-shaped pieces of shell placed into the sockets of three missing lower incisor teeth. For forty years the archaeological world considered that these shells were placed under the nose in a manner also observed in the ancient Egyptians. However, in 1970 a Brazilian dental academic, Professor Amadeo Bobbio studied the mandibular specimen and took a series of radiographs. He noted compact bone formation around two of the implants which led him to conclude that the implants were placed during life.
In the 1950s research was being conducted at Cambridge University in England to study blood flow in vivo. These workers devised a method of constructing a chamber of titanium which was then embedded into the soft tissue of the ears of rabbits. In 1952 the Swedish orthopaedic surgeon, P I Brånemark, was interested in studying bone healing and regeneration, and adopted the Cambridge designed ‘rabbit ear chamber’ for use in the rabbit femur. Following several months of study he attempted to retrieve these expensive chambers from the rabbits and found that he was unable to remove them. Per Brånemark observed that bone had grown into such close proximity with the titanium that it effectively adhered to the metal. Brånemark carried out many further studies into this phenomenon, using both animal and human subjects, which all confirmed this unique property of titanium.
Although he had originally considered that the first work should centre on knee and hip surgery, Brånemark finally decided that the mouth was more accessible for continued clinical observations and the high rate of edentulism in the general population offered more subjects for widespread study. He termed the clinically observed adherence of bone with titanium as ‘osseointegration’. In 1965 Brånemark, who was by then the Professor of Anatomy at Gothenburg University in Sweden, placed the first titanium dental implant into a human volunteer, a Swede named Gösta Larsson.
Over the next fourteen years Brånemark published many studies on the use of titanium in dental implantology until in 1978 he entered into a commercial partnership with the Swedish defense company, Bofors AB for the development and marketing of his dental implants. With Bofors (later to become Nobel Industries) as the parent company, Nobelpharma AB (later to be renamed Nobel Biocare) was founded in 1981 to focus on dental implantology. To the present day over 7 million Brånemark System implants have now been placed and hundreds of other companies produce dental implants. The majority of dental implants currently available are shaped like small screws, with either tapered or parallel sides. They can be placed at the same time as a tooth is removed by engaging with the bone of the socket wall and sometimes also with the bone beyond the tip of the socket. Current evidence suggests that implants placed straight into an extraction socket have comparable success rates to those placed into healed bone.[4]. The success rate and radiographic results of immediate restorations of dental implants placed in fresh extraction sockets (the temporary crowns placed at the same time) have been shown to be comparable to those obtained with delayed loading (the crowns placed weeks or months later)[5]
Some current research in dental implantology is focusing on the use of ceramic materials such as zirconia (ZrO2) in the manufacture of dental implants. Although generally the same shape as titanium implants zirconia, which has been used successfully for orthopaedic surgery for a number of years, has the advantage of being more cosmetically aesthetic owing to its bright tooth-like colour[6]. Long-term clinical data is necessary before one-piece ZrO2 implants can be recommended for daily practice[7].
Composition of Implants
A typical implant consists of a titanium screw (resembling a tooth root) with a roughened or smooth surface. The very first implants were made out of commercially pure titanium, however since it was discovered that the Ti6AlV4 alloy offered the same osseointegration level as commercially pure titanium, more and more implants were made out of Ti6AlV4 alloy due to its better tensile strength and thus fracture resistance. Today most implants are made out of the Ti6AlV4 alloy and treated either by plasma spraying, etching or sandblasting to increase the surface area and, thus the integration potential of the implant. An osteotomy or precision hole is carefully drilled into jawbone and the implant is installed in the osteotomy.
Training
Implant surgery is performed as an outpatient under general anesthesia (if several implants are to be placed) or with local anesthesia (for simple cases) by trained and certified clinicians including general dentists, oral surgeons, prosthodontists, and periodontists. An increasing number of cosmetic dentists are also placing implants in relatively simple cases. In the UK the General Dental Council has guidelines on the training required for a dentist to be able to place dental implants in general dental practice [8]. The degree to which both graduate and post-graduate dentists' receive training in the surgical placement of implants varies from country to country [9], [10], [11] but it seems clear that lack of formal training will lead to a higher complication rate [12]
Surgical Procedure
Surgical Planning
Prior to placement of the implant planning is required to indentify vital structure such as the inferior alveolar nerve or sinus and properly angle implants for the most esthetic outcome. Radiographs, usually a 'panorex' is exposed prior to the surgery. In some instances, a CT scan will be obtain and specialized CAD/CAM computer programs will be used to plan case.
Whether CT-guided or manual, a 'stent' is usually created to facilitate the placement of implants. A stent is an acrylic wafer that fits over the teeth or mucosa (when all the teeth are missing) with pre-drilled holes to show the position and angle of the implants to be placed.
Basic Procedure
In it's most basic form the placement of an osseointegrated implant requres a preparation into the bone using percision drills who's speed is tightly regulated [13] to prevent burning or pressure necrosis of the bone. After a variable amount of time to allow the bone to grow to the edge of the implant (osseointegration a tooth or teeth can be placed on the implant. The amount of time required to place an implant will vary depending on the experience of the practitioner and difficulty of the individual situation but it is typically 10min to 30min per implant.
Detail Procedure
At edentulous (without teeth) jaw sites, a pilot hole is bored into the recipient bone, taking care to avoid the vital structures (in particular the inferior alveolar nerve or IAN and the mental foramen within the mandible). Drilling into jawbone usually occurs in several separate steps. The pilot hole is expanded by using progressively wider drills (typically between three and seven successive drilling steps, depending on implant width and length). Care is taken not to damage the osteoblast or bone cells by overheating. A cooling saline spray keeps the temperature of the bone to below 47 degrees Celsius (approximately 117 degrees Fahrenheit). The implant screw can be self-tapping, and is screwed into place at a precise torque so as not to overload the surrounding bone (overloaded bone can die, a condition called osteonecrosis, which may lead to failure of the implant to fully integrate or bond with the jawbone). Typically in most implant systems, the osteotomy or drilled hole is about 1mm deeper than the implant being placed, due to the shape of the drill tip. Surgeons must take the added length into consideration when drilling in the vicinity of vital structures.
Surgical Incisions
Traditionally, an incision is made over the crest of the site where the implant is to be placed. This is referred to as a 'flap'. Some systems allow for 'flapless' surgery where a piece of mucosa is punched-out from over the implant site. Proponents of 'flapless' surgery believe that it decreases recovery while it's detractors believe it increases complication rates because the edge of bone cannot be visualized. [14], [15]
Healing Time
The amount of time required for an implant to become osseointegrated is a hotly debated topic.[16]. Consequently the amount of time that practitioners allow the implant to heal before placing a restoration on it varies widely. In general, practioners allow 2-6 months for healing but preliminary studies show that early loading of implant may not increase early or long term complications.[17]
1-Stage, 2-Stage Surgery
When an implant is placed either a healing abutment, which comes through the mucosa is placed or a 'cover screw' which is flush with the surface of the dental implant is placed. When a cover screw is placed the mucosa covers the implant while it integrates then a second surgery is completed to place the healing abutment.
2-Stage surgery is sometime choosen when a concurrent bone graft is placed or surgery on the mucosa may be required for esthetic reasons. Some implants are one piece so that no healing abutment is required.
In straight forward cases patients can be implanted and restored in a single surgery, in a procedure labeled "Immediate Loading". In such cases a provisional prosthetic tooth or crown is shaped to avoid the force of the bite transferring to the implant while it integrates with the bone.
Surgical Timing
There are different approaches to place dental implants after tooth extraction. The approaches are:
- Immediate post-extraction implant placement.
- Delayed immediate post-extraction implant placement (2 weeks to 3 months after extraction).
- Late implantation (3 months after tooth extraction).
According to the timing of loading of dental implants, the procedure of loading could be classified into:
- Immediate loading procedure.
- Early loading (1 week to 12 weeks).
- Staged loading (3-6 months).
- Late loading (more than 6 months).
Immediate Placement
An increasingly common strategy to preserve bone and reduce treatment times includes the placement of a dental implant into a recent extraction site. In addition, immediate loading is becoming more common as success rates for this procedure are now acceptable. This can cut months off the treatment time and in some cases a prosthetic tooth can be attached to the implants at the same time as the surgery to place the dental implants.
Most data suggests that when placed into single rooted tooth sites with healthy bone and mucosa around them, the success rates are comparable to that of delayed procedures with no additional complications. [18]
Use of CT Scanning
When computed tomography, also called cone beam computed tomography or CBCT (3D X-ray imaging) is used preoperatively to accurately pinpoint vital structures, the zone of safety may be reduced to 1 mm through the use of computer-aided design and production of a surgical drilling and angulation guide.
Complementary procedures
Sinus lifting is a common surgical intervention. A dentist or specialist with proper training such as an endodontist, periodontist, prosthodontist, or oral surgeon thickens the inadequate part of atrophic maxilla towards the sinus with the help of bone transplantation or bone expletive substance. This results in more volume for a better quality bone site for the implantation. Prudent clinicians who wish to avoid placement of implants into the sinus cavity pre-plan sinus lift surgery using the precision diagnostic guidance afforded by a 3D CBCT X-ray, as in the case of posterior mandibular implants discussed earlier.
Bone grafting will be necessary in cases where there is a lack of adequate maxillary or mandibular bone in terms of front to back (lip to tongue) depth or thickness; top to bottom height; and left to right width. Sufficient bone is needed in three dimensions to securely integrate with the root-like implant. Improved bone height -- which is very difficult to achieve -- is particularly important to assure ample anchorage of the implant's root-like shape because it has to support the mechanical stress of chewing, just like a natural tooth. If an implant is too shallow, chewing may cause a dangerous jawbone crack or full fracture.
Typically, implantologists try to place implants at least as deeply into bone as the crown or tooth will be above the bone. This is called a 1:1 crown to root ratio. This ratio establishes the target for bone grafting in most cases. If 1:1 or better cannot be achieved, the patient is usually advised that only a short implant can be placed and to not expect a long period of usability.
A wide range of grafting materials and substances may be used during the process of bone grafting / bone replacement. They include the patient's own bone (autograft), which may be harvested from the hip (iliac crest) or from spare jawbone; processed bone from cadavers (allograft); bovine bone or coral (xenograft); or artificially produced bonelike substances (calcium sulfate with names like Regeneform; and hydroxyapatite or HA, which is the primary form of calcium found in bone). The HA is effective as a substrate for osteoblasts to grow on. Some implants are coated with HA for this reason, although the bone forming properties of many of these substances is a hotly debated topic in bone research groups. Alternatively the bone intended to support the implant can be split and widened with the implant placed between the two havles like a sandwich. This is referred to as a 'ridge split' procedure..
Bone graft surgery has its own standard of care. In a typical procedure, the clinician creates a large flap of the gingiva or gum to fully expose the jawbone at the graft site, performs one or several types of block and onlay grafts in and on existing bone, then installs a membrane designed to repel unwanted infection-causing microbiota found in the oral cavity. Then the gingiva is carefully sutured over the site. Together with a course of internal antibiotics and external antibiotic mouth rinses, the graft site is allowed to heal (several months).
The clinician typically takes a new panoramic x-ray to confirm graft success in width and height, and assumes that positive signs in these two dimensions safely predicts success in the third dimension, depth. Where more precision is needed, usually when mandibular implants are being planned, a 3D or cone beam X-ray may be called for at this point to enable accurate measurement of bone and location of nerves and vital structures for proper treatment planning. The same X-ray data set can be employed for the preparation of computer-designed placement guides.
Correctly performed, a bone graft produces live vascular bone which is very much like natural jawbone and is therefore suitable as a foundation for implants.
Considerations
For dental implant procedure to work, there must be enough bone in the jaw, and the bone has to be strong enough to hold and support the implant. If there is not enough bone, more may need to be added with a bone graft procedure discussed earlier. Sometimes, this procedure is called bone augmentation. In addition, natural teeth and supporting tissues near where the implant will be placed must be in good health.
In all cases, what must be addressed is the functional aspect of the final implant restoration, the final occlusion. How much force per area is being placed on the bone implant interface? Implant loads from chewing and parafunction can exceed the physio biomechanic tolerance of the implant bone interface and/or the titanium material itself, causing failure. This can be failure of the implant itself (fracture) or bone loss, a "melting" or resorption of the surrounding bone.
The dentist must first determine what type of prosthesis will be fabricated. Only then can the specific implant requirements including number, length, diameter, and thread pattern be determined. In other words, the case must be reverse engineered by the restoring dentist prior to the surgery. If bone volume or density is inadequate, a bone graft procedure must be considered first. The restoring dentist may consult with the periodontist, endodontist, oral surgeon, or another trained general dentist to co-treat the patient. Usually, physical models or impressions of the patient's jawbones and teeth are made by the restorative dentist at the implant surgeons request, and are used as physical aids to treatment planning. If not supplied, the implant surgeon makes his own or relies upon advanced computer-assisted tomography or a cone beam CT scan to achieve the proper treatment plan.
Computer simulation software based on CT scan data allows virtual implant surgical placement based on a barium impregnated prototype of the final prosthesis. This predicts vital anatomy, bone quality, implant characteristics, the need for bone grafting, and maximizing the implant bone surface area for the treatment case creating a high level of predictability. Computer CAD/CAM milled or stereo lithography based drill guides can be developed for the implant surgeon to facilitate proper implant placement based on the final prosthesis occlusion and aesthetics.
Treatment planning software can also be used to demonstrate "try-ins" to the patient on a computer screen. When options have been fully discussed between patient and surgeon, the same software can be used to produce precision drill guides. A popular software package called Simplant (simulated implant) uses the digital data from a patient's CBCT to build a treatment plan, then produces a data set which is sent to a lab for production of a precision in-mouth drilling guide.[1]
Success rates
Dental implant success is related to operator skill, quality and quantity of the bone available at the site, and also to the patient's oral hygiene. The general consensus of opinion is that implants carry a success rate of around 95%[19]
Failure
Failure of a dental implant is often related to failure to osseointegrate correctly. A dental implant is considered to be a failure if it is lost, mobile or shows peri-implant (after implant) bone loss of greater than 1.0 mm in the first year and greater than 0.2mm a year after.
Dental implants are not susceptible to dental caries but they can develop a periodontal condition called peri-implantitis. The cause may be infection that was introduced during surgery; or failure by the patient to follow correct oral hygiene routines. In either case, inflammation in the bone surrounding the implant causes bone loss (recession) which ultimately may lead to failure, often evidenced by the ability to "spin" an implant.
Peri-implantitis is often dealt with pre-emptively by clinicians who prescribe a course of antibiotics in the days prior to surgery; and post-surgically with another course of antibiotics and special oral rinses. Since peri-implantitis is generally easy to see on standard panoramic and periapical X-rays, prudent clinicians who suspect the problem will take an X-ray soon after surgery, and again at staged intervals post-operatively.
Risk of failure is increased in smokers. For this reason implants are frequently placed only after a patient has stopped smoking as the treatment is very expensive. More rarely, an implant may fail because of poor positioning at the time of surgery, or may be overloaded initially causing failure to integrate. If smoking and positioning problems exist prior to implant surgery, clinicians often advise patients that a bridge or partial denture rather than an implant may be a better solution.
Failure may also occur independently of the causes outlined above. Implants like any other object suffers from wear and tear. If the implants in question are replacing commonly used teeth, then these may suffer from wear and tear and after years may crack and break up. This is a very rare occurrence, however possible. The only way to 'avoid' or prolong this from happening is to visit your dentist frequently.
Contraindications
There are no absolute contraindications to implant dentistry, however there are some systemic, behavioral and anatomic considerations that should be considered.
Particularly for mandibular (lower jaw) implants, especially in the vicinity of the mental foramen (MF), there must be sufficient alveolar bone above the mandibular canal also called the inferior alveolar canal or IAC (which acts as the conduit for the neurovascular bundle carrying the inferior alveolar nerve or IAN).
Failure to precisely locate the IAN and MF invites surgical insult by the drills and the implant itself. Such insult may cause irreparable damage to the nerve, often felt as a paresthesia (numbness) or dysesthesia (painful numbness) of the gum, lip and chin. This condition may persist for life and may be accompanied by unconscious drooling.
Lack of sufficient alveolar bone is another contraindication to the procedure. Typically, a preoperative in-office panoramic X-ray is taken to establish (with allowances for image distortion, a known problem with panoramic X-rays) in two dimensions (height and width) the amount of available bone. A bone graft or augmentation procedure may be performed and allowed to heal several months before implantation surgery. A new panoramic X-ray will help determine if the graft was successful.
This is an important step inasmuch as improved bone height is much more difficult to achieve than more increased bone depth. For mandibular grafts, helical cone beam computed tomography (CBCT) enables measurement of bone height (top to bottom), width (left and right) and depth (front to back) to an accuracy of 0.1mm or better. The precision of cone beam has stimulated a new industry that produces computer-designed surgical guides based on the cone beam X-ray's digital data. These surgery aids are employed by implantologists to precisely locate and drill into the mandible and maxilla, and to avoid vital structures.
Uncontrolled type II diabetes is a significant relative contraindication as healing following any type of surgical procedure is delayed due to poor peripheral blood circulation. Anatomic considerations include the volume and height of bone available. Often an ancillary procedure known as a block graft or sinus augmentation are needed to provide enough bone for successful implant placement.
There is new information about intravenous and oral bisphosphonates (taken for certain forms of breast cancer and osteoporosis, respectively) which may put patients at a higher risk of developing a delayed healing syndrome called osteonecrosis. Implants are contraindicated for some patients who take intravenous bisphosphonates.
The many millions of patients who take an oral bisphosphonate (such as Actonel, Fosamax and Boniva) may be advised to stop the administration prior to implant surgery, then resume several months later. But this protocol may not be necessary. As of January, 2008, an oral bisphosphonate study reported in the February 2008 Journal of Oral and Maxillofacial Surgery, reviewing 115 cases that included 468 implants, concluded "There is no evidence of bisphosphonate-associated osteonecrosis of the jaw in any of the patients evaluated in the clinic and those contacted by phone or e-mail reported no symptoms." (JOMS, Volume 66, Issue 2, Ppgs 223-230).
The American Dental Association had addressed bisphosphonates in an article entitled "Bisphosphonate medications and your oral health," (JADA, Vol. 137, page 1048, July 2006.) In an Overview, the ADA stated "The risk of developing BON [bisphosphonate-associated osteonecrosis of the jaw] in patients on oral bisphosphonate therapy appears to be very low...". The ADA Council on Scientific Affairs also employed a panel of experts who issued recommendations [for clinicians] for treatment of patients on oral bisphosphonates, published in June, 2006. The overview may be read online at ada.org but it has now been superseded by a huge study -- encompassing over 700,000 cases -- entitled "Bisphosphonate Use and the Risk of Adverse Jaw Outcomes." Like the 2008 JOMS study, the ADA study exonerates oral bisphosphonates as a contraindication to dental implants. (JADA, January 2008, 139:23-30).
Bruxism (tooth clenching or grinding) is another contraindication. The forces generated during bruxism are particularly detrimental to implants while bone is healing; micromovements in the implant positioning are associated with increased rates of implant failure. Bruxism continues to pose a threat to implants throughout the life of the recipient. Natural teeth contain a periodontal ligament allowing each tooth to move and absorb shock in response to vertical and horizontal forces. Once replaced by dental implants, this ligament is lost and teeth are immovably anchored directly into the jaw bone. This problem can be minimized by wearing a custom made mouthguard (such an NTI appliance) at night.
Postoperatively, after implants have been placed, there are physical contraindications that prompt rapid action by the implantology team. Excessive or severe pain lasting more than three days is a warning sign, as is excessive bleeding. Constant numbness of the gingiva (gum), lip and chin -- usually noticed after surgical anesthesia wears off -- is another warning sign. In the latter case, which may be accompanied by severe constant pain, the standard of care calls for diagnosis to determine if the surgical procedure insulted the IAN. A 3D cone beam X-ray provides the necessary data, but even before this step a prudent implantologist may back out or completely remove an implant in an effort to restore nerve function because delay is usually ineffective. Depending upon the evidence visible with a 3D X-ray, patients may be referred to a specialist in nerve repair. In all cases, speed in diagnosis and treatment are necessary.
The market
In the United States, implantology is not a recognized specialty however it is a recognized specialty in Germany. Various ‘implant surgeons’ play a role in the placement of dental implants. Specialists with adequate comprehensive training such as Prosthodontists are ideally preferred. Other specialists such as periodontists, oral surgeons, and endodontists participate in the placement of implants. Also, some general dentists trained and skilled in implant surgery may place dental implants. However caution is warranted as a rigorous training cannot be substituted with a weekend course. It is almost always necessary for advanced bone surgical skills (such as grafting) to be employed during implant treatment. Therefore, it is important for the ‘implant surgeon’ to be able to have surgical options available, through adequate training, to serve the needs of patients. It is common for implant care to be coordinated amongst the designated ‘implant surgeon’ and the dentist for the placement of the implant and restoration of the implant with a tooth, teeth, or some other form of a prosthesis such as an implant supported over-denture or perhaps a hybrid prosthesis.
Notes
- ^ James RA. Subperiosteal implant design based on peri-implant tissue behavior. N Y J Dent 1983;53:407-414
- ^ Linkow LI, Kohen PA. Benefits and risks of the endosteal blade implant (Harvard Conference, June 1978). J Oral Implantol 1980;9:9-44
- ^ Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977;16:1-132
- ^ How does the timing of implant placement to extraction affect outcome? Int J Oral Maxillofac Implants. 2007;22 Suppl:203-23.
- ^ Immediate versus delayed loading of dental implants placed in fresh extraction sockets in the maxillary esthetic zone: a clinical comparative study. Int J Oral Maxillofac Implants. 2008 Jul-Aug;23(4):753-8.
- ^ Osseointegration of zirconia implants: an SEM observation of the bone-implant interface. Head Face Med. 2008 Nov 6;4:25.
- ^ Fracture Strength of Zirconia Implants after Artificial Aging. Clin Implant Dent Relat Res. 2008 Jul 23.
- ^ Doing implants? Make sure you’re up to scratch, warns GDC. general Dental Council Press Release, Thursday, October 30, 2008
- ^ Melo MD, McGann G, Obeid G. J Oral Maxillofac Surg. 2007 Dec;65(12):2554-8.
- ^ Jokstad A. Int J Oral Maxillofac Surg. 2008 Jul;37(7):593-6. Epub 2008 Mar 4.
- ^ Addy LD, Lynch CD, Locke M, Watts A, Gilmour AS. Br Dent J. 2008 Dec 13;205(11):609-14.
- ^ Binon PP. J Oral Maxillofac Surg. 2007 Jul;65(7 Suppl 1):73-92. Erratum in: J Oral Maxillofac Surg. 2008 Oct;66(10):2195-6.
- ^ Brisman DL. Int J Oral Maxillofac Implants. 1996 Jan-Feb;11(1):35-7.
- ^ Berdougo M, Fortin T, Blanchet E, Isidori M, Bosson JL. Assistant professor, Department of Periodontology, Dental University of Lyon, France.
- ^ Becker W, Goldstein M, Becker BE, Sennerby L, Kois D, Hujoel P. J Periodontol. 2009 Feb;80(2):347-352.
- ^ Gerds TA, Vogeler M. Stat Methods Med Res. 2005 Dec;14(6):579-90.
- ^ Fischer K, Stenberg T, Hedin M, Sennerby L. Clin Oral Implants Res. 2008 May;19(5):433-41. Epub 2008 Mar 26.
- ^ Bhola M, Neely AL, Kolhatkar S. J Prosthodont. 2008 Oct;17(7):576-81. Epub 2008 Aug 26. Review.
- ^ The effectiveness of immediate, early, and conventional loading of dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2007 Nov-Dec;22(6):893-904.
See also
- Periodontist
- Oral and maxillofacial surgery
- Bone grafts in Dental Implantology
- Dental bridge
- Dental tourism
External links
Associations and organizations
- Osteonecrosis overview at the American Dental Association
- OsseoNews Expert Discussions on Dental Implants
- American Academy of Implant Dentistry
- Academy of Osseointegration
- ITI:International Team for Implantology
- American Association of Oral and Maxillofacial Surgeons
- American Academy of Periodontology
- Association of Dental Implantology UK
- European Association for Osseointegration
- American Dental Implant Association
- International Congress of Oral Implantologists
- British Society of Oral Implantology
- Dental Implants Before and After
Publications
