Heterosis

Heterosis, hybrid vigor, or outbreeding enhancement is the improved or increased function of any biological quality in a hybrid offspring. An offspring is heterotic if its traits are enhanced as a result of mixing the genetic contributions of its parents. These effects can be due to Mendelian or non-Mendelian inheritance.
Definitions
In proposing the term heterosis to replace the older term heterozygosis, G.H. Shull aimed to avoid limiting the term to the effects that can be explained by heterozygosity in Mendelian inheritance.[1]
The physiological vigor of an organism as manifested in its rapidity of growth, its height and general robustness, is positively correlated with the degree of dissimilarity in the gametes by whose union the organism was formed … The more numerous the differences between the uniting gametes — at least within certain limits — the greater on the whole is the amount of stimulation … These differences need not be Mendelian in their inheritance … To avoid the implication that all the genotypic differences which stimulate cell-division, growth and other physiological activities of an organism are Mendelian in their inheritance and also to gain brevity of expression I suggest … that the word 'heterosis' be adopted.
Heterosis is often discussed as the opposite of inbreeding depression, although differences in these two concepts can be seen in evolutionary considerations such as the role of genetic variation or the effects of genetic drift in small populations on these concepts. Inbreeding depression occurs when related parents have children with traits that negatively influence their fitness largely due to homozygosity. In such instances, outcrossing should result in heterosis.
Not all outcrosses result in heterosis. For example, when a hybrid inherits traits from its parents that are not fully compatible, fitness can be reduced. This is a form of outbreeding depression, the effects of which are similar to inbreeding depression.[2]
Genetic and epigenetic bases
Since the early 1900s, two competing genetic hypotheses, not necessarily mutually exclusive, have been developed to explain hybrid vigor. More recently, an epigenetic component of hybrid vigor has also been established.[3][4]
Dominance and overdominance
When a population is small or inbred, it tends to lose genetic diversity. Inbreeding depression is the loss of fitness due to loss of genetic diversity. Inbred strains tend to be homozygous for recessive alleles that are mildly harmful (or produce a trait that is undesirable from the standpoint of the breeder). Heterosis or hybrid vigor, on the other hand, is the tendency of outbred strains to exceed both inbred parents in fitness.
Selective breeding of plants and animals, including hybridization, began long before there was an understanding of underlying scientific principles. In the early 20th century, after Mendel's laws came to be understood and accepted, geneticists undertook to explain the superior vigor of many plant hybrids. Two competing hypotheses, which are not mutually exclusive, were developed:[5]
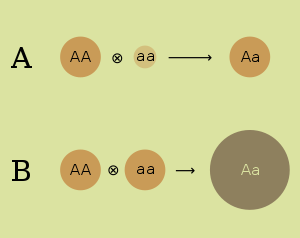
- Dominance hypothesis. The dominance hypothesis attributes the superiority of hybrids to the suppression of undesirable recessive alleles from one parent by dominant alleles from the other. It attributes the poor performance of inbred strains to loss of genetic diversity, with the strains becoming purely homozygous at many loci. The dominance hypothesis was first expressed in 1908 by the geneticist Charles Davenport.[6] Under the dominance hypothesis, deleterious alleles are expected to be maintained in a random-mating population at a selection–mutation balance that would depend on the rate of mutation, the effect of the alleles and the degree to which alleles are expressed in heterozygotes.[7]
- Overdominance hypothesis. Certain combinations of alleles that can be obtained by crossing two inbred strains are advantageous in the heterozygote. The overdominance hypothesis attributes the heterozygote advantage to the survival of many alleles that are recessive and harmful in homozygotes. It attributes the poor performance of inbred strains to a high percentage of these harmful recessives. The overdominance hypothesis was developed independently by Edward M. East (1908)[8] and George Shull (1908).[9] Genetic variation at an overdominant locus is expected to be maintained by balancing selection. The high fitness of heterozygous genotypes favours the persistence of an allelic polymorphism in the population.[7] This hypothesis is commonly invoked to explain the persistence of some alleles (most famously the Sickle cell trait allele) that are harmful in homozygotes. In normal circumstances, such harmful alleles would be removed from a population through the process of natural selection. Like the dominance hypothesis, it attributes the poor performance of inbred strains to expression of such harmful recessive alleles.
Dominance and overdominance have different consequences for the gene expression profile of the individuals. If overdominance is the main cause for the fitness advantages of heterosis, then there should be an over-expression of certain genes in the heterozygous offspring compared to the homozygous parents. On the other hand, if dominance is the cause, fewer genes should be under-expressed in the heterozygous offspring compared to the parents. Furthermore, for any given gene, the expression should be comparable to the one observed in the fitter of the two parents. In any case, outcross matings provide the benefit of masking deleterious recessive alleles in progeny. This benefit has been proposed to be a major factor in the maintenance of sexual reproduction among eukaryotes, as summarized in the article Evolution of sexual reproduction.
Historical retrospective
Which of the two mechanisms are the "main" reason for hetrosis has been a scientific controversy in the field of genetics.[10] Population geneticist James Crow (1916–2012) believed, in his younger days, that overdominance was a major contributor to hybrid vigor. In 1998 he published a retrospective review of the developing science.[11] According to Crow, the demonstration of several cases of heterozygote advantage in Drosophila and other organisms first caused great enthusiasm for the overdominance theory among scientists studying plant hybridization. But overdominance implies that yields on an inbred strain should decrease as inbred strains are selected for the performance of their hybrid crosses, as the proportion of harmful recessives in the inbred population rises. Over the years, experimentation in plant genetics has proven that the reverse occurs, that yields increase in both the inbred strains and the hybrids, suggesting that dominance alone may be adequate to explain the superior yield of hybrids. Only a few conclusive cases of overdominance have been reported in all of genetics. Since the 1980s, as experimental evidence has mounted, the dominance theory has made a comeback.
Crow wrote:
The current view ... is that the dominance hypothesis is the major explanation of inbreeding decline and [of] the high yield of hybrids. There is little statistical evidence for contributions from overdominance and epistasis. But whether the best hybrids are getting an extra boost from overdominance or favorable epistatic contributions remains an open question.[11]
Epigenetics
An epigenetic contribution to heterosis has been established in plants,[4] and it has also been reported in animals.[12] MicroRNAs (miRNAs), discovered in 1993, are a class of non-coding small RNAs which repress the translation of messenger RNAs (mRNAs) or cause degradation of mRNAs.[13] In hybrid plants, most miRNAs have non-additive expression (it might be higher or lower than the levels in the parents).[4] This suggests that the small RNAs are involved in the growth, vigor and adaptation of hybrids.[4]
'Heterosis without hybridity' effects on plant size have been demonstrated in genetically isogenic F1 triploid (autopolyploid) plants, where paternal genome excess F1 triploids display positive heterosis, whereas maternal genome excess F1s display negative heterosis effects.[14] Such findings demonstrate that heterosis effects, with a genome dosage-dependent epigenetic basis, can be generated in F1 offspring that are genetically isogenic (i.e. harbour no heterozygosity).[14][15] It has been shown[3] that hybrid vigor in an allopolyploid hybrid of two Arabidopsis species was due to epigenetic control in the upstream regions of two genes, which caused major downstream alteration in chlorophyll and starch accumulation. The mechanism involves acetylation and/or methylation of specific amino acids in histone H3, a protein closely associated with DNA, which can either activate or repress associated genes.
Specific mechanisms
Major histocompatibility complex in animals
One example of where particular genes may be important in vertebrate animals for heterosis is the major histocompatibility complex (MHC). Vertebrates inherit several copies of both MHC class I and MHC class II from each parent, which are used in antigen presentation as part of the adaptive immune system. Each different copy of the genes is able to bind and present a different set of potential peptides to T-lymphocytes. These genes are highly polymorphic throughout populations, but are more similar in smaller, more closely related populations. Breeding between more genetically distant individuals decreases the chance of inheriting two alleles that are the same or similar, allowing a more diverse range of peptides to be presented. This, therefore, gives a decreased chance that any particular pathogen will not be recognised, and means that more antigenic proteins on any pathogen are likely to be recognised, giving a greater range of T-cell activation, so a greater response. This also means that the immunity acquired to the pathogen is against a greater range of antigens, meaning that the pathogen must mutate more before immunity is lost. Thus, hybrids are less likely to succumb to pathogenic disease and are more capable of fighting off infection. This may be the cause, though, of autoimmune diseases.[citation needed]
Plants
Crosses between inbreds from different heterotic groups result in vigorous F1 hybrids with significantly more heterosis than F1 hybrids from inbreds within the same heterotic group or pattern. Heterotic groups are created by plant breeders to classify inbred lines, and can be progressively improved by reciprocal recurrent selection.
Heterosis is used to increase yields, uniformity, and vigor. Hybrid breeding methods are used in maize, sorghum, rice, sugar beet, onion, spinach, sunflowers, broccoli and to create a more psychoactive cannabis.
Corn (maize)
Nearly all field corn (maize) grown in most developed nations exhibits heterosis. Modern corn hybrids substantially outyield conventional cultivars and respond better to fertilizer.
Corn heterosis was famously demonstrated in the early 20th century by George H. Shull and Edward M. East after hybrid corn was invented by Dr. William James Beal of Michigan State University based on work begun in 1879 at the urging of Charles Darwin. Dr. Beal's work led to the first published account of a field experiment demonstrating hybrid vigor in corn, by Eugene Davenport and Perry Holden, 1881. These various pioneers of botany and related fields showed that crosses of inbred lines made from a Southern dent and a Northern flint, respectively, showed substantial heterosis and outyielded conventional cultivars of that era. However, at that time such hybrids could not be economically made on a large scale for use by farmers. Donald F. Jones at the Connecticut Agricultural Experiment Station, New Haven invented the first practical method of producing a high-yielding hybrid maize in 1914–1917. Jones' method produced a double-cross hybrid, which requires two crossing steps working from four distinct original inbred lines. Later work by corn breeders produced inbred lines with sufficient vigor for practical production of a commercial hybrid in a single step, the single-cross hybrids. Single-cross hybrids are made from just two original parent inbreds. They are generally more vigorous and also more uniform than the earlier double-cross hybrids. The process of creating these hybrids often involves detasseling.
Temperate maize hybrids are derived from two main heterotic groups: 'Iowa Stiff Stalk Synthetic', and nonstiff stalk.[citation needed]
Rice (Oryza sativa)
Rice production has seen enormous rise in China due to heavy uses of hybrid rice. In China, efforts have generated a super hybrid rice strain ('LYP9') with a production capability around 15 tons per hectare. In India also, several varieties have shown high vigor, including 'RH-10' and 'Suruchi 5401'.
Animals
Hybrid livestock
The concept of heterosis is also applied in the production of commercial livestock. In cattle, crosses between Black Angus and Hereford produce a cross known as a "Black Baldy". In swine, "blue butts" are produced by the cross of Hampshire and Yorkshire. Other, more exotic hybrids (two different species, so genetically more dissimilar), such as "beefalo" which are hybrids of cattle and bison, are also used for specialty markets.
Poultry
Within poultry, sex-linked genes have been used to create hybrids in which males and females can be sorted at one day old by color. Specific genes used for this are genes for barring and wing feather growth. Crosses of this sort create what are sold as Black Sex-links, Red Sex-links, and various other crosses that are known by trade names.
Commercial broilers are produced by crossing different strains of White Rocks and White Cornish, the Cornish providing a large frame and the Rocks providing the fast rate of gain. The hybrid vigor produced allows the production of uniform birds at a marketable carcass weight at 6–9 weeks of age.
Likewise, hybrids between different strains of White Leghorn are used to produce laying flocks that provide the majority of white eggs for sale in the United States.
Dogs
In 2013, a study found that mixed breeds live on average 1.2 years longer than pure breeds.[16]
John Scott and John L. Fuller performed a detailed study of purebred Cocker Spaniels, purebred Basenjis, and hybrids between them.[17] They found that hybrids ran faster than either parent, perhaps due to heterosis. Other characteristics, such as basal heart rate, did not show any heterosis—the dog's basal heart rate was close to the average of its parents—perhaps due to the additive effects of multiple genes.[18]
Sometimes people working on a dog-breeding program find no useful heterosis.[19]
All this said, studies do not provide definitive proof of hybrid vigor in dogs. This is largely due to the unknown heritage of most mixed breeds mutts used. Results vary wildly, with some studies showing benefit and others finding the mixed breed dogs to be more prone to genetic conditions.[20][21][22]
Birds
In 2014, a study undertaken by the Centre for Integrative Ecology at Deakin University in Geelong, Victoria, concluded that intraspecific hybrids between the subspecies Platycercus elegans flaveolus and P. e. elegans of the crimson rosella (P. elegans) were more likely to fight off diseases than their pure counterparts.[23]
Humans
Human beings are all extremely genetically similar to one another.[24][25][26] Michael Mingroni has proposed heterosis, in the form of hybrid vigor associated with historical reductions of the levels of inbreeding, as an explanation of the Flynn effect, the steady rise in IQ test scores around the world during the 20th century, [citation needed] though a review of nine studies found that there is no evidence to suggest inbreeding has an effect on IQ.[27]
Controversy
The term heterosis often causes confusion and even controversy, particularly in selective breeding of domestic animals, because it is sometimes (incorrectly) claimed that all crossbred plants and animals are "genetically superior" to their parents, due to heterosis,[citation needed]. but two problems exist with this claim:
- First, according to an article published in the journal Genome Biology, "genetic superiority" is an ill-defined term and not generally accepted terminology within the scientific field of genetics.[28] A related term fitness is well defined, but it can rarely be directly measured. Instead, scientists use objective, measurable quantities, such as the number of seeds a plant produces, the germination rate of a seed, or the percentage of organisms that survive to reproductive age.[29] From this perspective, crossbred plants and animals exhibiting heterosis may have "superior" traits, but this does not necessarily equate to any evidence of outright "genetic superiority". Use of the term "superiority" is commonplace for example in crop breeding, where it is well understood to mean a better-yielding, more robust plant for agriculture. Such a plant may yield better on a farm, but would likely struggle to survive in the wild, making this use open to misinterpretation. In human genetics any question of "genetic superiority" is even more problematic due to the historical and political implications of any such claim. Some may even go as far as to describe it as a questionable value judgement in the realm of politics, not science.[28]
- Second, not all hybrids exhibit heterosis (see outbreeding depression).
An example of the ambiguous value judgements imposed on hybrids and hybrid vigor is the mule. While mules are almost always infertile, they are valued for a combination of hardiness and temperament that is different from either of their horse or donkey parents. While these qualities may make them "superior" for particular uses by humans, the infertility issue implies that these animals would most likely become extinct without the intervention of humans through animal husbandry, making them "inferior" in terms of natural selection.
See also
References
- ^ George Harrison Shull (1948). "What Is "Heterosis"?". Genetics. 33 (5): 439–446. doi:10.1093/genetics/33.5.439. PMC 1209417. PMID 17247290.
- ^ Edmands, Suzanne (2006-11-15). "Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management: RELATIVE RISKS OF INBREEDING AND OUTBREEDING". Molecular Ecology. 16 (3): 463–475. doi:10.1111/j.1365-294X.2006.03148.x.
- ^ a b Ni Z, Kim ED, Ha M, et al. (January 2009). "Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids". Nature. 457 (7227): 327–31. Bibcode:2009Natur.457..327N. doi:10.1038/nature07523. PMC 2679702. PMID 19029881.
- ^ a b c d Baranwal VK, Mikkilineni V, Zehr UB, Tyagi AK, Kapoor S (November 2012). "Heterosis: emerging ideas about hybrid vigour". J. Exp. Bot. 63 (18): 6309–14. doi:10.1093/jxb/ers291. PMID 23095992.
- ^ Crow, James F. (1948). "Alternative Hypotheses of Hybrid Vigor". Genetics. 33 (5): 477–487. doi:10.1093/genetics/33.5.477. PMC 1209419. PMID 17247292.
- ^ Davenport CB (1908). "Degeneration, albinism and inbreeding". Science. 28 (718): 454–5. Bibcode:1908Sci....28..454D. doi:10.1126/science.28.718.454-b. PMID 17771943. S2CID 29068462.
- ^ a b Carr, David E.; Dudash, Michele R. (2003-06-29). "Recent approaches into the genetic basis of inbreeding depression in plants". Philosophical Transactions of the Royal Society B: Biological Sciences. 358 (1434): 1071–1084. doi:10.1098/rstb.2003.1295. ISSN 0962-8436. PMC 1693197. PMID 12831473.
- ^ East EM (1908). "Inbreeding in corn". Reports of the Connecticut Agricultural Experiments Station for 1907: 419–428.
- ^ Shull GH (1908). "The composition of a field of maize". Reports of the American Breeders Association: 296–301.
- ^ Birchler J.A.; Auger D.L.; Riddle N.C. (2003). "In search of the molecular basis of heterosis". The Plant Cell. 15 (10): 2236–2239. doi:10.1105/tpc.151030. PMC 540269. PMID 14523245.
- ^ a b Crow, James F. (1998). "90 Years Ago: The Beginning of Hybrid Maize". Genetics. 148 (3): 923–928. doi:10.1093/genetics/148.3.923. PMC 1460037. PMID 9539413.
- ^ Han Z, Mtango NR, Patel BG, Sapienza C, Latham KE (October 2008). "Hybrid vigor and transgenerational epigenetic effects on early mouse embryo phenotype". Biol. Reprod. 79 (4): 638–48. doi:10.1095/biolreprod.108.069096. PMC 2844494. PMID 18562704.
- ^ Zhou Y, Ferguson J, Chang JT, Kluger Y (2007). "Inter- and intra-combinatorial regulation by transcription factors and microRNAs". BMC Genomics. 8: 396. doi:10.1186/1471-2164-8-396. PMC 2206040. PMID 17971223.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Fort, Antoine; Ryder, Peter; McKeown, Peter C.; Wijnen, Cris; Aarts, Mark G.; Sulpice, Ronan; Spillane, Charles (2016-01-01). "Disaggregating polyploidy, parental genome dosage and hybridity contributions to heterosis in Arabidopsis thaliana". The New Phytologist. 209 (2): 590–599. doi:10.1111/nph.13650. ISSN 1469-8137. PMID 26395035.
- ^ Duszynska, Dorota; McKeown, Peter C.; Juenger, Thomas E.; Pietraszewska-Bogiel, Anna; Geelen, Danny; Spillane, Charles (2013-04-01). "Gamete fertility and ovule number variation in selfed reciprocal F1 hybrid triploid plants are heritable and display epigenetic parent-of-origin effects". The New Phytologist. 198 (1): 71–81. doi:10.1111/nph.12147. ISSN 1469-8137. PMID 23368793.
- ^ O'Neill, D. G.; Church, D. B.; McGreevy, P. D.; Thomson, P. C.; Brodbelt, D. C. (2013). "Longevity and mortality of owned dogs in England" (PDF). The Veterinary Journal. 198 (3): 638–43. doi:10.1016/j.tvjl.2013.09.020. PMID 24206631.
- ^ Spady, Tyrone C.; Ostrander, Elaine A. (2008). "Canine Behavioral Genetics: Pointing Out the Phenotypes and Herding up the Genes". American Journal of Human Genetics. 82 (1): 10–8. doi:10.1016/j.ajhg.2007.12.001. PMC 2253978. PMID 18179880.
- ^ John Paul Scott and John L. Fuller. "Genetics and the Social Behavior of the Dog". 1965. p. 307 and p. 313.
- ^ Per Jensen. "The Behavioural Biology of Dogs". 2007. p. 179
- ^ Nicholas, Frank W (2016). "Hybrid vigour in dogs?". Veterinary Journal. 214. National Library of Medicine: 77–83. doi:10.1016/j.tvjl.2016.05.013. PMID 27387730. Retrieved 29 July 2020.
- ^ Nicholas, J A C (2012). "Survey of ophthalmic abnormalities in the labradoodle in the UK". The Veterinary Record. 170 (15). National Library of Medicine: 390. doi:10.1136/vr.100361. PMID 22278634. S2CID 5932838. Retrieved 29 July 2020.
- ^ Sharkey, Laura (2020). "Hybrid Vigor in Dogs". The Functional Dog Collaborative. The Functional Dog Collaborative. Retrieved 29 July 2020.
- ^ Australian Geographic (September 2014). "Hybrid birds better at fighting disease than purebreds".
- ^ Hawks, John (2013). Significance of Neandertal and Denisovan Genomes in Human Evolution. Vol. 42. Annual Reviews. pp. 433–449, 438. doi:10.1146/annurev-anthro-092412-155548. ISBN 978-0-8243-1942-7. ISSN 0084-6570.
The shared evolutionary history of living humans has resulted in a high relatedness among all living people, as indicated for example by the very low fixation index (FST) among living human populations.
{{cite book}}:|journal=ignored (help) - ^ Barbujani, Guido; Colonna, Vincenza (15 September 2011). "Chapter 6: Genetic Basis of Human Biodiversity: An Update". In Zachos, Frank E.; Habel, Jan Christian (eds.). Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. Springer. pp. 97–119. doi:10.1007/978-3-642-20992-5_6. ISBN 978-3-642-20992-5. Retrieved 23 November 2013.
The massive efforts to study the human genome in detail have produced extraordinary amounts of genetic data. Although we still fail to understand the molecular bases of most complex traits, including many common diseases, we now have a clearer idea of the degree of genetic resemblance between humans and other primate species. We also know that humans are genetically very close to each other, indeed more than any other primates, that most of our genetic diversity is accounted for by individual differences within populations, and that only a small fraction of the species' genetic variance falls between populations and geographic groups thereof.
- ^ Ramachandran, Sohini; Tang, Hua; Gutenkunst, Ryan N.; Bustamante, Carlos D. (2010). "Chapter 20: Genetics and Genomics of Human Population Structure". In Speicher, Michael R.; Antonarakis, Stylianos E.; Motulsky, Arno G. (eds.). Vogel and Motulsky's Human Genetics: Problems and Approaches. Heidelberg: Springer Scientific. pp. 589–615. doi:10.1007/978-3-540-37654-5_22. ISBN 978-3-540-37653-8. S2CID 89318627.
Most studies of human population genetics begin by citing a seminal 1972 paper by Richard Lewontin bearing the title of this subsection [29]. Given the central role this work has played in our field, we will begin by discussing it briefly and return to its conclusions throughout the chapter. ... A key conclusion of the paper is that 85.4% of the total genetic variation observed occurred within each group. That is, he reported that the vast majority of genetic differences are found within populations rather than between them. ... His finding has been reproduced in study after study up through the present: two random individuals from any one group (which could be a continent or even a local population) are almost as different as any two random individuals from the entire world
- ^ Kamin, Leon J. (1980). "Inbreeding depression and IQ". Psychological Bulletin. 87 (3): 469–478. doi:10.1037/0033-2909.87.3.469. ISSN 1939-1455.
- ^ a b Risch N, Burchard E, Ziv E, Tang H (July 2002). "Categorization of humans in biomedical research: genes, race and disease". Genome Biol. 3 (7): comment2007. doi:10.1186/gb-2002-3-7-comment2007. PMC 139378. PMID 12184798.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Weller SG, Sakai AK, Thai DA, Tom J, Rankin AE (November 2005). "Inbreeding depression and heterosis in populations of Schiedea viscosa, a highly selfing species". J. Evol. Biol. 18 (6): 1434–44. doi:10.1111/j.1420-9101.2005.00965.x. PMID 16313456.
Further reading
- Bakker, Winfridus (2006). "Enhanced Hybrid Vigor Benefits Breeder and Broiler" (PDF). Cobb Focus (2). Archived from the original (PDF) on 2008-12-17.
- Birchler JA, Auger DL, Riddle NC (October 2003). "In Search of the Molecular Basis of Heterosis". Plant Cell. 15 (10): 2236–9. doi:10.1105/tpc.151030. PMC 540269. PMID 14523245.
- NOAA Tech Memo NMFS NWFSC-30: Genetic Effects of Straying of Non-Native Hatchery Fish into Natural Populations: Inbreeding Depression and Outbreeding Depression
- Mac Gregor, S. E. (2009) [1976]. "Introduction: Hybrid vigor in plants and its relationship to insect pollination" (PDF). Insect Pollination of Cultivated Crop Plants. Agriculture Handbook no. 496. Agricultural Research Service, U.S.D.A. OCLC 243509268.
- "Hybrids & Heirlooms"—an article from University of Illinois Extension's Home Hort Hints
- Roybal, J. (July 1, 1998). "Ranchstar". Beef (beefmagazine.com).
- "Sex-Links"—regarding poultry; at FeatherSite
