Super-resolution microscopy
A major contributor to this article appears to have a close connection with its subject. (May 2020) |
Super-resolution microscopy is a series of techniques in optical microscopy that allow such images to have resolutions higher than those imposed by the diffraction limit,[1][2] which is due to the diffraction of light.[3] Super-resolution imaging techniques rely on the near-field (photon-tunneling microscopy[4] as well as those that use the Pendry Superlens and near field scanning optical microscopy) or on the far-field. Among techniques that rely on the latter are those that improve the resolution only modestly (up to about a factor of two) beyond the diffraction-limit, such as confocal microscopy with closed pinhole or aided by computational methods such as deconvolution[5] or detector-based pixel reassignment (e.g. re-scan microscopy,[6] pixel reassignment[7]), the 4Pi microscope, and structured-illumination microscopy technologies such as SIM[8][9] and SMI.
There are two major groups of methods for super-resolution microscopy in the far-field that can improve the resolution by a much larger factor:[10]
- Deterministic super-resolution: the most commonly used emitters in biological microscopy, fluorophores, show a nonlinear response to excitation, which can be exploited to enhance resolution. Such methods include STED, GSD, RESOLFT and SSIM.
- Stochastic super-resolution: the chemical complexity of many molecular light sources gives them a complex temporal behavior, which can be used to make several nearby fluorophores emit light at separate times and thereby become resolvable in time. These methods include super-resolution optical fluctuation imaging (SOFI) and all single-molecule localization methods (SMLM), such as SPDM, SPDMphymod, PALM, FPALM, STORM, and dSTORM.
On 8 October 2014, the Nobel Prize in Chemistry was awarded to Eric Betzig, W.E. Moerner and Stefan Hell for "the development of super-resolved fluorescence microscopy", which brings "optical microscopy into the nanodimension".[11][12] The different modalities of super-resolution microscopy are increasingly being adopted by the biomedical research community, and these techniques are becoming indispensable tools to understanding biological function at the molecular level.[13]
History
[edit]By 1978, the first theoretical ideas had been developed to break the Abbe limit, which called for using a 4Pi microscope as a confocal laser-scanning fluorescence microscope where the light is focused from all sides to a common focus that is used to scan the object by 'point-by-point' excitation combined with 'point-by-point' detection.[14] However the publication from 1978 [15] had drawn an improper physical conclusion (i.e. a point-like spot of light) and had completely missed the axial resolution increase as the actual benefit of adding the other side of the solid angle.[16]
Some of the following information was gathered (with permission) from a chemistry blog's review of sub-diffraction microscopy techniques.[17][18]
In 1986, a super-resolution optical microscope based on stimulated emission was patented by Okhonin.[19]
Super-resolution techniques
[edit]This section is empty. You can help by adding to it. (May 2020) |
Local enhancement / ANSOM / optical nano-antennas
[edit]This section is empty. You can help by adding to it. (May 2020) |
Near-field optical random mapping (NORM) microscopy
[edit]Near-field optical random mapping (NORM) microscopy is a method of optical near-field acquisition by a far-field microscope through the observation of nanoparticles' Brownian motion in an immersion liquid.[21][22]
NORM uses object surface scanning by stochastically moving nanoparticles. Through the microscope, nanoparticles look like symmetric round spots. The spot width is equivalent to the point spread function (~ 250 nm) and is defined by the microscope resolution. Lateral coordinates of the given particle can be evaluated with a precision much higher than the resolution of the microscope. By collecting the information from many frames one can map out the near field intensity distribution across the whole field of view of the microscope. In comparison with NSOM and ANSOM this method does not require any special equipment for tip positioning and has a large field of view and a depth of focus. Due to the large number of scanning "sensors" one can achieve image acquisition in a shorter time.
4Pi
[edit]A 4Pi microscope is a laser-scanning fluorescence microscope with an improved axial resolution. The typical value of 500–700 nm can be improved to 100–150 nm, which corresponds to an almost spherical focal spot with 5–7 times less volume than that of standard confocal microscopy.
The improvement in resolution is achieved by using two opposing objective lenses, both of which are focused to the same geometric location. Also, the difference in optical path length through each of the two objective lenses is carefully minimized. By this, molecules residing in the common focal area of both objectives can be illuminated coherently from both sides, and the reflected or emitted light can be collected coherently, i.e. coherent superposition of emitted light on the detector is possible. The solid angle that is used for illumination and detection is increased and approaches the ideal case, where the sample is illuminated and detected from all sides simultaneously.[23][24]
Up to now, the best quality in a 4Pi microscope has been reached in conjunction with STED microscopy in fixed cells[25] and RESOLFT microscopy with switchable proteins in living cells.[26]
Structured illumination microscopy (SIM)
[edit]
Structured illumination microscopy (SIM) enhances spatial resolution by collecting information from frequency space outside the observable region. This process is done in reciprocal space: the Fourier transform (FT) of an SI image contains superimposed additional information from different areas of reciprocal space; with several frames where the illumination is shifted by some phase, it is possible to computationally separate and reconstruct the FT image, which has much more resolution information. The reverse FT returns the reconstructed image to a super-resolution image.
SIM could potentially replace electron microscopy as a tool for some medical diagnoses. These include diagnosis of kidney disorders,[27] kidney cancer,[28] and blood diseases.[29]
Although the term "structured illumination microscopy" was coined by others in later years, Guerra (1995) first published results[30] in which light patterned by a 50 nm pitch grating illuminated a second grating of pitch 50 nm, with the gratings rotated with respect to each other by the angular amount needed to achieve magnification. Although the illuminating wavelength was 650 nm, the 50 nm grating was easily resolved. This showed a nearly 5-fold improvement over the Abbe resolution limit of 232 nm that should have been the smallest obtained for the numerical aperture and wavelength used. In further development of this work, Guerra showed that super-resolved lateral topography is attained by phase-shifting the evanescent field. Several U.S. patents[31] were issued to Guerra individually, or with colleagues, and assigned to the Polaroid Corporation. Licenses to this technology were procured by Dyer Energy Systems, Calimetrics Inc., and Nanoptek Corp. for use of this super-resolution technique in optical data storage and microscopy.
- Images of cell nuclei and mitotic stages recorded with 3D-SIM.
-
Comparison confocal microscopy – 3D-SIM
-
Cell nucleus in prophase from various angles
-
Two mouse cell nuclei in prophase.
-
mouse cell in telophase
Spatially modulated illumination (SMI)
[edit]
One implementation of structured illumination is known as spatially modulated illumination (SMI). Like standard structured illumination, the SMI technique modifies the point spread function (PSF) of a microscope in a suitable manner. In this case however, "the optical resolution itself is not enhanced";[32] instead structured illumination is used to maximize the precision of distance measurements of fluorescent objects, to "enable size measurements at molecular dimensions of a few tens of nanometers".[32]
The Vertico SMI microscope achieves structured illumination by using one or two opposing interfering laser beams along the axis. The object being imaged is then moved in high-precision steps through the wave field, or the wave field itself is moved relative to the object by phase shifts. This results in an improved axial size and distance resolution.[32][33][34]
SMI can be combined with other super resolution technologies, for instance with 3D LIMON or LSI-TIRF as a total internal reflection interferometer with laterally structured illumination (this last instrument and technique is essentially a phase-shifted photon tunneling microscope, which employs a total internal reflection light microscope with phase-shifted evanescent field (Guerra, 1996).[31] This SMI technique allows one to acquire light-optical images of autofluorophore distributions in sections from human eye tissue with previously unmatched optical resolution. Use of three different excitation wavelengths (488, 568, and 647 nm), enables one to gather spectral information about the autofluorescence signal. This has been used to examine human eye tissue affected by macular degeneration.[35]
Biosensing
[edit]Biosensing is crucial for understanding the activities of cellular components in cell biology. Genetically encoded sensors have transformed this field and typically consist of two parts: the sensing domain, which detects cellular activity or interactions, and the reporting domain, which produces measurable signals. There are two main types of sensors: FRET-based sensors using two fluorophores for precise quantification but with some limitations, and single-fluorophore biosensors that are smaller, faster, and allow for multiplexed experiments, but may have challenges in obtaining absolute values and detecting response saturation. Various microscopy methods, including super-resolution optical fluctuation imaging, have been used to quantify and monitor biological activities in real time. Examples include calcium, pH, and voltage sensing. Greenwald et al. offer a more comprehensive overview of these applications.[36]
Deterministic functional techniques
[edit]REversible Saturable OpticaL Fluorescence Transitions (RESOLFT) microscopy is an optical microscopy with very high resolution that can image details in samples that cannot be imaged with conventional or confocal microscopy. Within RESOLFT the principles of STED microscopy[37][38] and GSD microscopy are generalized. Also, there are techniques with other concepts than RESOLFT or SSIM. For example, fluorescence microscopy using the optical AND gate property of nitrogen-vacancy center,[39] or super-resolution by Stimulated Emission of Thermal Radiation (SETR), which uses the intrinsic super-linearities of the Black-Body radiation and expands the concept of super-resolution beyond microscopy.[40]
Stimulated emission depletion (STED)
[edit]
Stimulated emission depletion microscopy (STED) uses two laser pulses, the excitation pulse for excitation of the fluorophores to their fluorescent state and the STED pulse for the de-excitation of fluorophores by means of stimulated emission.[19][41][42][43][44][45] In practice, the excitation laser pulse is first applied whereupon a STED pulse soon follows (STED without pulses using continuous wave lasers is also used). Furthermore, the STED pulse is modified in such a way so that it features a zero-intensity spot that coincides with the excitation focal spot. Due to the non-linear dependence of the stimulated emission rate on the intensity of the STED beam, all the fluorophores around the focal excitation spot will be in their off state (the ground state of the fluorophores). By scanning this focal spot, one retrieves the image. The full width at half maximum (FWHM) of the point spread function (PSF) of the excitation focal spot can theoretically be compressed to an arbitrary width by raising the intensity of the STED pulse, according to equation (1).
- (1)
- where ∆r is the lateral resolution, ∆ is the FWHM of the diffraction limited PSF, Imax is the peak intensity of the STED laser, and is the threshold intensity needed in order to achieve saturated emission depletion.
The main disadvantage of STED, which has prevented its widespread use, is that the machinery is complicated. On the one hand, the image acquisition speed is relatively slow for large fields of view because of the need to scan the sample in order to retrieve an image. On the other hand, it can be very fast for smaller fields of view: recordings of up to 80 frames per second have been shown.[46][47] Due to a large Is value associated with STED, there is the need for a high-intensity excitation pulse, which may cause damage to the sample.
Ground state depletion (GSD)
[edit]Ground state depletion microscopy (GSD microscopy) uses the triplet state of a fluorophore as the off-state and the singlet state as the on-state, whereby an excitation laser is used to drive the fluorophores at the periphery of the singlet state molecule to the triplet state. This is much like STED, where the off-state is the ground state of fluorophores, which is why equation (1) also applies in this case. The value is smaller than in STED, making super-resolution imaging possible at a much smaller laser intensity. Compared to STED, though, the fluorophores used in GSD are generally less photostable; and the saturation of the triplet state may be harder to realize.[48]
Saturated structured illumination microscopy (SSIM)
[edit]Saturated structured-illumination microscopy (SSIM) exploits the nonlinear dependence of the emission rate of fluorophores on the intensity of the excitation laser.[49] By applying a sinusoidal illumination pattern[50] with a peak intensity close to that needed in order to saturate the fluorophores in their fluorescent state, one retrieves Moiré fringes. The fringes contain high order spatial information that may be extracted by computational techniques. Once the information is extracted, a super-resolution image is retrieved.
SSIM requires shifting the illumination pattern multiple times, effectively limiting the temporal resolution of the technique. In addition there is the need for very photostable fluorophores, due to the saturating conditions, which inflict radiation damage on the sample and restrict the possible applications for which SSIM may be used.
Examples of this microscopy are shown under section Structured illumination microscopy (SIM): images of cell nuclei and mitotic stages recorded with 3D-SIM Microscopy.
Stochastic functional techniques
[edit]Localization microscopy
[edit]Single-molecule localization microscopy (SMLM) summarizes all microscopical techniques that achieve super-resolution by isolating emitters and fitting their images with the point spread function (PSF). Normally, the width of the point spread function (~ 250 nm) limits resolution. However, given an isolated emitter, one is able to determine its location with a precision only limited by its intensity according to equation (2).[51]
- (2)
- where Δloc is the localization precision, Δ is the FWHM (full width at half maximum) of the PSF and N is the number of collected photons.
This fitting process can only be performed reliably for isolated emitters (see Deconvolution), and interesting biological samples are so densely labeled with emitters that fitting is impossible when all emitters are active at the same time. SMLM techniques solve this dilemma by activating only a sparse subset of emitters at the same time, localizing these few emitters very precisely, deactivating them and activating another subset.
Considering background and camera pixelation, and using Gaussian approximation for the point spread function (Airy disk) of a typical microscope, the theoretical resolution is proposed by Thompson et al.[52] and fine-tuned by Mortensen et al.:[53]
- where
- * σ is the Gaussian standard deviation of the center locations of the same molecule if measured multiple times (e.g. frames of a video). (unit m)
- * σPSF is the Gaussian standard deviation of the point spread function, whose FWHM following the Ernst Abbe equation d = λ/(2 N.A.). (unit m)
- * a is the size of each image pixel. (unit m)
- * Nsig is the photon counts of the total PSF over all pixels of interest. (unitless)
- * Nbg the average background photon counts per pixel (dark counts already removed), which is approximated to be the square of the Gaussian standard deviation of the Poisson distribution background noise of each pixel over time or standard deviation of all pixels with background noise only, σbg2. The larger the σbg2, the better the approximation (e.g. good for σbg2 >10, excellent for σbg2 >1000). (unitless)
- * Resolution FWHM is ~2.355 times the Gaussian standard deviation.
Generally, localization microscopy is performed with fluorophores. Suitable fluorophores (e.g. for STORM) reside in a non-fluorescent dark state for most of the time and are activated stochastically, typically with an excitation laser of low intensity. A readout laser stimulates fluorescence and bleaches or photoswitches the fluorophores back to a dark state, typically within 10–100 ms. In Points Accumulation for Imaging in Nanoscale Topography (PAINT), the fluorophores are nonfluorescent before binding and afterwards become fluorescent. The photons emitted during the fluorescent phase are collected with a camera and the resulting image of the fluorophore (which is distorted by the PSF) can be fitted with very high precision, even on the order of a few Angstroms.[54] Repeating the process several thousand times ensures that all fluorophores can go through the bright state and are recorded. A computer then reconstructs a super-resolved image.
The desirable traits of fluorophores used for these methods, in order to maximize the resolution, are that they should be bright. That is, they should have a high extinction coefficient and a high quantum yield. They should also possess a high contrast ratio (ratio between the number of photons emitted in the light state and the number of photons emitted in the dark state). Also, a densely labeled sample is desirable, according to the Nyquist criteria.
The multitude of localization microscopy methods differ mostly in the type of fluorophores used.
Spectral precision distance microscopy (SPDM)
[edit]
A single, tiny source of light can be located much better than the resolution of a microscope usually allows for: although the light will produce a blurry spot, computer algorithms can be used to accurately calculate the center of the blurry spot, taking into account the point spread function of the microscope, the noise properties of the detector, etc. However, this approach does not work when there are too many sources close to each other: the sources then all blur together.
Spectral precision distance microscopy (SPDM) is a family of localizing techniques in fluorescence microscopy which gets around the problem of there being many sources by measuring just a few sources at a time, so that each source is "optically isolated" from the others (i.e., separated by more than the microscope's resolution, typically ~200-250 nm).[55][56][57] This "optical isolation" requires that the particles under examination have different spectral signatures, so that it is possible to look at light from just a few molecules at a time by using the appropriate light sources and filters. This achieves an effective optical resolution several times better than the conventional optical resolution that is represented by the half-width of the main maximum of the effective point image function.[55]
The structural resolution achievable using SPDM can be expressed in terms of the smallest measurable distance between two punctiform particles of different spectral characteristics ("topological resolution"). Modeling has shown that under suitable conditions regarding the precision of localization, particle density, etc., the "topological resolution" corresponds to a "space frequency" that, in terms of the classical definition, is equivalent to a much improved optical resolution. Molecules can also be distinguished in even more subtle ways based on fluorescent lifetime and other techniques.[55]
An important application is in genome research (study of the functional organization of the genome). Another important area of use is research into the structure of membranes.
SPDMphymod
[edit]
Localization microscopy for many standard fluorescent dyes like GFP, Alexa dyes, and fluorescein molecules is possible if certain photo-physical conditions are present. With this so-called physically modifiable fluorophores (SPDMphymod) technology, a single laser wavelength of suitable intensity is sufficient for nanoimaging[58] in contrast to other localization microscopy technologies that need two laser wavelengths when special photo-switchable/photo-activatable fluorescence molecules are used. A further example of the use of SPDMphymod is an analysis of Tobacco mosaic virus (TMV) particles[59] or the study of virus–cell interaction.[60][61]
Based on singlet–triplet state transitions it is crucial for SPDMphymod that this process is ongoing and leading to the effect that a single molecule comes first into a very long-living reversible dark state (with half-life of as much as several seconds) from which it returns to a fluorescent state emitting many photons for several milliseconds before it returns into a very long-living, so-called irreversible dark state. SPDMphymod microscopy uses fluorescent molecules that emit the same spectral light frequency but with different spectral signatures based on the flashing characteristics. By combining two thousands images of the same cell, it is possible, using laser optical precision measurements, to record localization images with significantly improved optical resolution.[62]
Standard fluorescent dyes already successfully used with the SPDMphymod technology are GFP, RFP, YFP, Alexa 488, Alexa 568, Alexa 647, Cy2, Cy3, Atto 488 and fluorescein.
Cryogenic optical localization in 3D (COLD)
[edit]
Cryogenic Optical Localization in 3D (COLD) is a method that allows localizing multiple fluorescent sites within a single small- to medium-sized biomolecule with Angstrom-scale resolution.[54] The localization precision in this approach is enhanced because the slower photochemistry at low temperatures leads to a higher number of photons that can be emitted from each fluorophore before photobleaching.[63][64] As a result, cryogenic stochastic localization microscopy achieves the sub-molecular resolution required to resolve the 3D positions of several fluorophores attached to a small protein. By employing algorithms known from electron microscopy, the 2D projections of fluorophores are reconstructed into a 3D configuration. COLD brings fluorescence microscopy to its fundamental limit, depending on the size of the label. The method can also be combined with other structural biology techniques—such as X-ray crystallography, magnetic resonance spectroscopy, and electron microscopy—to provide valuable complementary information and specificity.
Binding-activated localization microscopy (BALM)
[edit]
Binding-activated localization microscopy (BALM) is a general concept for single-molecule localization microscopy (SMLM): super-resolved imaging of DNA-binding dyes based on modifying the properties of DNA and a dye.[65] By careful adjustment of the chemical environment—leading to local, reversible DNA melting and hybridization control over the fluorescence signal—DNA-binding dye molecules can be introduced. Intercalating and minor-groove binding DNA dyes can be used to register and optically isolate only a few DNA-binding dye signals at a time. DNA structure fluctuation-assisted BALM (fBALM) has been used to nanoscale differences in nuclear architecture, with an anticipated structural resolution of approximately 50 nm. Imaging chromatin nanostructure with binding-activated localization microscopy based on DNA structure fluctuations.[66] Recently, the significant enhancement of fluorescence quantum yield of NIAD-4 upon binding to an amyloid was exploited for BALM imaging of amyloid fibrils[67] and oligomers.[68]
STORM, PALM, and FPALM
[edit]Stochastic optical reconstruction microscopy (STORM), photo activated localization microscopy (PALM), and fluorescence photo-activation localization microscopy (FPALM) are super-resolution imaging techniques that use sequential activation and time-resolved localization of photoswitchable fluorophores to create high resolution images. During imaging, only an optically resolvable subset of fluorophores is activated to a fluorescent state at any given moment, such that the position of each fluorophore can be determined with high precision by finding the centroid positions of the single-molecule images of a particular fluorophore. One subset of fluorophores is subsequently deactivated, and another subset is activated and imaged. Iteration of this process allows numerous fluorophores to be localized and a super-resolution image to be constructed from the image data.
These three methods were published independently over a short period of time, and their principles are identical. STORM was originally described using Cy5 and Cy3 dyes attached to nucleic acids or proteins,[69] while PALM and FPALM were described using photoswitchable fluorescent proteins.[70][71] In principle any photoswitchable fluorophore can be used, and STORM has been demonstrated with a variety of different probes and labeling strategies. Using stochastic photoswitching of single fluorophores, such as Cy5,[72] STORM can be performed with a single red laser excitation source. The red laser both switches the Cy5 fluorophore to a dark state by formation of an adduct[73][74] and subsequently returns the molecule to the fluorescent state. Many other dyes have been also used with STORM.[75][76][77][78][79][80]
In addition to single fluorophores, dye-pairs consisting of an activator fluorophore (such as Alexa 405, Cy2, or Cy3) and a photoswitchable reporter dye (such as Cy5, Alexa 647, Cy5.5, or Cy7) can be used with STORM.[69][81][82] In this scheme, the activator fluorophore, when excited near its absorption maximum, serves to reactivate the photoswitchable dye to the fluorescent state. Multicolor imaging has been performed by using different activation wavelengths to distinguish dye-pairs, depending on the activator fluorophore used,[81][82][83] or using spectrally distinct photoswitchable fluorophores, either with or without activator fluorophores.[75][84][85] Photoswitchable fluorescent proteins can be used as well.[70][71][85][86] Highly specific labeling of biological structures with photoswitchable probes has been achieved with antibody staining,[81][82][83][87] direct conjugation of proteins,[88] and genetic encoding.[70][71][85][86]
STORM has also been extended to three-dimensional imaging using optical astigmatism, in which the elliptical shape of the point spread function encodes the x, y, and z positions for samples up to several micrometers thick,[82][87] and has been demonstrated in living cells.[85] To date, the spatial resolution achieved by this technique is ~20 nm in the lateral dimensions and ~50 nm in the axial dimension; and the temporal resolution is as fast as 0.1–0.33s.[citation needed]
Points accumulation for imaging in nanoscale topography (PAINT)
[edit]Points accumulation for imaging in nanoscale topography (PAINT) is a single-molecule localization method that achieves stochastic single-molecule fluorescence by molecular adsorption/absorption and photobleaching/desorption.[89][90] The first dye used was Nile red which is nonfluorescent in aqueous solution but fluorescent when inserted into a hydrophobic environment, such as micelles or living cell walls. Thus, the concentration of the dye is kept small, at the nanomolar level, so that the molecule's sorption rate to the diffraction-limited area is in the millisecond region. The stochastic binding of single-dye molecules (probes) to an immobilized target can be spatially and temporally resolved under a typical widefield fluorescence microscope. Each dye is photobleached to return the field to a dark state, so the next dye can bind and be observed. The advantage of this method, compared to other stochastic methods, is that in addition to obtaining the super-resolved image of the fixed target, it can measure the dynamic binding kinetics of the diffusing probe molecules, in solution, to the target.[91][90]
Combining 3D super-resolution technique (e.g. the double-helix point spread function develop in Moerner's group), photo-activated dyes, power-dependent active intermittency, and points accumulation for imaging in nanoscale topography, SPRAIPAINT (SPRAI=Super resolution by PoweR-dependent Active Intermittency[92]) can super-resolve live-cell walls.[93] PAINT works by maintaining a balance between the dye adsorption/absorption and photobleaching/desorption rates. This balance can be estimated with statistical principles.[94] The adsorption or absorption rate of a dilute solute to a surface or interface in a gas or liquid solution can be calculated using Fick's laws of diffusion. The photobleaching/desorption rate can be measured for a given solution condition and illumination power density.
DNA-PAINT has been further extended to use regular dyes, where the dynamic binding and unbinding of a dye-labeled DNA probe to a fixed DNA origami is used to achieve stochastic single-molecule imaging.[95][96] DNA-PAINT is no longer limited to environment-sensitive dyes and can measure both the adsorption and the desorption kinetics of the probes to the target. The method uses the camera blurring effect of moving dyes. When a regular dye is diffusing in the solution, its image on a typical CCD camera is blurred because of its relatively fast speed and the relatively long camera exposure time, contributing to the fluorescence background. However, when it binds to a fixed target, the dye stops moving; and clear input into the point spread function can be achieved.
The term for this method is mbPAINT ("mb" standing for motion blur).[97] When a total internal reflection fluorescence microscope (TIRF) is used for imaging, the excitation depth is limited to ~100 nm from the substrate, which further reduces the fluorescence background from the blurred dyes near the substrate and the background in the bulk solution. Very bright dyes can be used for mbPAINT which gives typical single-frame spatial resolutions of ~20 nm and single-molecule kinetic temporal resolutions of ~20 ms under relatively mild photoexcitation intensities, which is useful in studying molecular separation of single proteins.[98]
The temporal resolution has been further improved (20 times) using a rotational phase mask placed in the Fourier plane during data acquisition and resolving the distorted point spread function that contains temporal information. The method was named Super Temporal-Resolved Microscopy (STReM).[99]
Label-free localization microscopy
[edit]
Optical resolution of cellular structures in the range of about 50 nm can be achieved, even in label-free cells, using localization microscopy SPDM.
By using two different laser wavelengths, SPDM reveals cellular objects which are not detectable under conventional fluorescence wide-field imaging conditions, beside making for a substantial resolution improvement of autofluorescent structures.
As a control, the positions of the detected objects in the localization image match those in the bright-field image.[100]
Label-free superresolution microscopy has also been demonstrated using the fluctuations of a surface-enhanced Raman scattering signal on a highly uniform plasmonic metasurface.[101]
Direct stochastical optical reconstruction microscopy (dSTORM)
[edit]dSTORM uses the photoswitching of a single fluorophore. In dSTORM, fluorophores are embedded in a reducing and oxidizing buffering system (ROXS) and fluorescence is excited. Sometimes, stochastically, the fluorophore will enter a triplet or some other dark state that is sensitive to the oxidation state of the buffer, from which they can be made to fluoresce, so that single molecule positions can be recorded.[102] Development of the dSTORM method occurred at 3 independent laboratories at about the same time and was also called "reversible photobleaching microscopy" (RPM),[103] "ground state depletion microscopy followed by individual molecule return" (GSDIM),[104] as well as the now generally accepted moniker dSTORM.[105]
Software for localization microscopy
[edit]Localization microscopy depends heavily on software that can precisely fit the point spread function (PSF) to millions of images of active fluorophores within a few minutes.[106] Since the classical analysis methods and software suites used in the natural sciences are too slow to computationally solve these problems, often taking hours of computation for processing data measured in minutes, specialised software programs have been developed. Many of these localization software packages are open-source; they are listed at SMLM Software Benchmark.[107] Once molecule positions have been determined, the locations need to be displayed and several algorithms for display have been developed.[108]
Super-resolution optical fluctuation imaging (SOFI)
[edit]It is possible to circumvent the need for PSF fitting inherent in single molecule localization microscopy (SMLM) by directly computing the temporal autocorrelation of pixels. This technique is called super-resolution optical fluctuation imaging (SOFI) and has been shown to be more precise than SMLM when the density of concurrently active fluorophores is very high.
Omnipresent Localization Microscopy (OLM)
[edit]Omnipresent Localisation Microscopy (OLM) is an extension of Single Molecule Microscopy (SMLM) techniques that allow high-density single molecule imaging with an incoherent light source (such as a mercury-arc lamp) and a conventional epifluorescence microscope setup.[109] A short burst of deep-blue excitation (with a 350-380 nm, instead of a 405 nm, laser) enables a prolonged reactivation of molecules, for a resolution of 90 nm on test specimens. Finally, correlative STED and SMLM imaging can be performed on the same biological sample using a simple imaging medium, which can provide a basis for a further enhanced resolution. These findings can democratize super-resolution imaging and help any scientist to generate high-density single-molecule images even with a limited budget.
Resolution Enhancement by Sequential Imaging (RESI)
[edit]Resolution enhancement by sequential imaging (RESI) is an extension of DNA-PAINT that can achieve theoretically unlimited resolution.[110] Rather than using one label type to identify a given target species, copies of the same target are labeled with orthogonal DNA sequences. Upon sequential (i.e. separated) imaging, localization clouds that would overlap in conventional SMLM can be (1) resolved and (2) combined into a single "super" localization, the precision of which scales with the underlying number of localizations. As the number of achievable localizations in DNA-PAINT is unlimited, so is the theoretical resolution of RESI. Overlaying the RESI localizations from the underlying imaging rounds creates a composite, highly resolved image.
Combination of techniques
[edit]3D light microscopical nanosizing (LIMON) microscopy
[edit]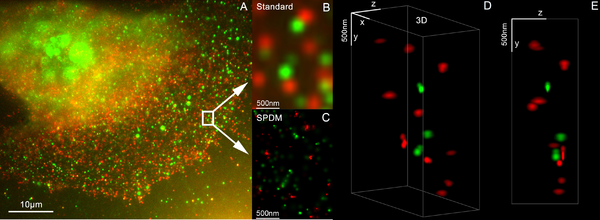
Light MicrOscopical Nanosizing microscopy (3D LIMON) images, using the Vertico SMI microscope, are made possible by the combination of SMI and SPDM, whereby first the SMI, and then the SPDM, process is applied.
The SMI process determines the center of particles and their spread in the direction of the microscope axis. While the center of particles/molecules can be determined with a precision of 1–2 nm, the spread around this point can be determined down to an axial diameter of approximately 30–40 nm.
Subsequently, the lateral position of the individual particle/molecule is determined using SPDM, achieving a precision of a few nanometers.[111]
As a biological application in the 3D dual color mode, the spatial arrangements of Her2/neu and Her3 clusters was achieved. The positions in all three directions of the protein clusters could be determined with an accuracy of about 25 nm.[112]
Integrated correlative light and electron microscopy
[edit]Combining a super-resolution microscope with an electron microscope enables the visualization of contextual information, with the labelling provided by fluorescence markers. This overcomes the problem of the black backdrop that the researcher is left with when using only a light microscope. In an integrated system, the sample is measured by both microscopes simultaneously.[113]
Enhancing of techniques using neural networks
[edit]Recently, owing to advancements in artificial intelligence computing, deep learning neural networks (GANs) have been used for super-resolution enhancing of photographic images extracted from optical microscopes,[114] enhancing resolution from 40x to 100x.[115] Resolution increases from 20x with an optical microscope to 1500x, comparable to a scanning electron microscope, via a neural lens.[116] These techniques have applications in super-resolving images from positron-emission tomography and fluorescence microscopy.[117]
See also
[edit]- Correlative light-electron microscopy
- Deconvolution
- Multifocal plane microscopy (MUM)
- Photoactivatable probes
- Photoactivated localization microscopy (PALM)
- Stimulated emission depletion microscope (STED)
- Super-resolution imaging
- Video super resolution
References
[edit]- ^ Neice A (2010). Methods and Limitations of Subwavelength Imaging. Advances in Imaging and Electron Physics. Vol. 163. pp. 117–140. doi:10.1016/S1076-5670(10)63003-0. ISBN 978-0-12-381314-5.
- ^ Stockert JC, Blázquez-Castro A (2017). "Chapter 20 Super-resolution Microscopy". Fluorescence Microscopy in Life Sciences. Bentham Science Publishers. pp. 687–711. ISBN 978-1-68108-519-7. Archived from the original on 14 May 2019. Retrieved 24 December 2017.
- ^ Abbe E (1873). "Beitrage zur Theorie des Mikroskops und der mikroskopischen Wahrmehmung" (PDF). Archiv für mikroskopische Anatomie (in German). 9: 413–420. doi:10.1007/BF02956173. S2CID 135526560.
- ^ Guerra JM (September 1990). "Photon tunneling microscopy". Applied Optics. 29 (26): 3741–52. Bibcode:1990ApOpt..29.3741G. doi:10.1364/AO.29.003741. PMID 20567479. S2CID 23505916.
- ^ Agard DA, Sedat JW (April 1983). "Three-dimensional architecture of a polytene nucleus". Nature. 302 (5910): 676–81. Bibcode:1983Natur.302..676A. doi:10.1038/302676a0. PMID 6403872. S2CID 4311047.
- ^ De Luca GM, Breedijk RM, Brandt RA, Zeelenberg CH, de Jong BE, Timmermans W, et al. (1 November 2013). "Re-scan confocal microscopy: scanning twice for better resolution". Biomedical Optics Express. 4 (11): 2644–56. doi:10.1364/BOE.4.002644. PMC 3829557. PMID 24298422.
- ^ Sheppard CJ, Mehta SB, Heintzmann R (August 2013). "Superresolution by image scanning microscopy using pixel reassignment". Optics Letters. 38 (15): 2889–92. Bibcode:2013OptL...38.2889S. doi:10.1364/OL.38.002889. hdl:1912/6208. PMID 23903171.
- ^ Guerra, John M. (26 June 1995). "Super-resolution through illumination by diffraction-born evanescent waves". Applied Physics Letters. 66 (26): 3555–3557. Bibcode:1995ApPhL..66.3555G. doi:10.1063/1.113814. ISSN 0003-6951.
- ^ U.S. Pat. No. 5,666,197: Apparatus and methods employing phase control and analysis of evanescent illumination for imaging and metrology of subwavelength lateral surface topography; John M. Guerra, September 1997. Assigned to Polaroid Corp.
- ^ SPIE (March 2015). "W.E. Moerner plenary presentation: Single-molecule spectroscopy, imaging, and photocontrol -- foundations for super-resolution microscopy". SPIE Newsroom. doi:10.1117/2.3201503.17.
- ^ Ritter K, Rising M (8 October 2014). "2 Americans, 1 German win chemistry Nobel". Associated Press. Retrieved 8 October 2014.
- ^ Chang K (8 October 2014). "2 Americans and a German Are Awarded Nobel Prize in Chemistry". The New York Times. Retrieved 8 October 2014.
- ^ Vangindertael, J.; Camacho, R.; Sempels, W.; Mizuno, H.; Dedecker, P.; Janssen, K. P. F. (2018). "An introduction to optical super-resolution microscopy for the adventurous biologist". Methods and Applications in Fluorescence. 6 (2): 022003. Bibcode:2018MApFl...6b2003V. doi:10.1088/2050-6120/aaae0c. ISSN 2050-6120. PMID 29422456.
- ^ Cremer C, Cremer T (September 1978). "Considerations on a laser-scanning-microscope with high resolution and depth of field". Microscopica Acta. 81 (1): 31–44. PMID 713859.
- ^ C. Cremer and T. Cremer (1978): Considerations on a laser-scanning-microscope with high resolution and depth of field Microscopica Acta VOL. 81 NUMBER 1 September, pp. 31—44 (1978)
- ^ The Nobel Prize in Chemistry 2014 https://www.nobelprize.org/prizes/chemistry/2014/hell/biographical/
- ^ Part I and Part II
- ^ Moerner WE (2006). "Single-molecule mountains yield nanoscale cell images". Nature Methods. 3 (10): 781–782. doi:10.1038/nmeth1006-781. PMC 2663419. PMID 16990808.
- ^ a b V.A. Okhonin, Method of investigating specimen microstructure, Patent SU 1374922, priority date 10 April 1986, Published on July 30, 1991, Soviet Patents Abstracts, Section EI, Week 9218, Derwent Publications Ltd., London, GB; Class S03, p. 4. Cited by patents US 5394268 A (1993) and US RE38307 E1 (1995). From the English translation: "The essence of the invention is as follows. Luminescence is excited in a sample placed in the field of several standing light waves, which cause luminescence quenching because of stimulated transitions...".
- ^ Guerra, John M. (10 September 1990). "Photon tunneling microscopy". Applied Optics. 29 (26): 3741–3752. Bibcode:1990ApOpt..29.3741G. doi:10.1364/AO.29.003741. ISSN 2155-3165. PMID 20567479.
- ^ US patent 2009/0116,024, priority date 7 April 2006: J. V. Mikliaev, S. A. Asselborn Method for obtaining a high resolution image
- ^ Miklyaev YV, Asselborn SA, Zaytsev KA, Darscht MY (2014). "Superresolution microscopy in far-field by near-field optical random mapping nanoscopy". Appl. Phys. Lett. 105 (11): 113103(1–4). Bibcode:2014ApPhL.105k3103M. doi:10.1063/1.4895922.
- ^ Cremer C, Cremer T (1978). "Considerations on a laser-scanning-microscope with high resolution and depth of field" (PDF). Microscopica Acta. 81 (1): 31–44. PMID 713859. Archived from the original (PDF) on 4 March 2016. Retrieved 22 May 2011.
- ^ Hell SW, Stelzer EH, Lindek S, Cremer C (February 1994). "Confocal microscopy with an increased detection aperture: type-B 4Pi confocal microscopy". Optics Letters. 19 (3): 222. Bibcode:1994OptL...19..222H. CiteSeerX 10.1.1.501.598. doi:10.1364/OL.19.000222. PMID 19829598.
- ^ Schmidt R, Wurm CA, Jakobs S, Engelhardt J, Egner A, Hell SW (June 2008). "Spherical nanosized focal spot unravels the interior of cells". Nature Methods. 5 (6): 539–44. doi:10.1038/nmeth.1214. hdl:11858/00-001M-0000-0012-DBBB-8. PMID 18488034. S2CID 16580036.
- ^ Böhm U, Hell SW, Schmidt R (February 2016). "4Pi-RESOLFT nanoscopy". Nature Communications. 7 (10504): 10504. Bibcode:2016NatCo...710504B. doi:10.1038/ncomms10504. PMC 4740410. PMID 26833381.
- ^ Pullman JM, Nylk J, Campbell EC, Gunn-Moore FJ, Prystowsky MB, Dholakia K (February 2016). "Visualization of podocyte substructure with structured illumination microscopy (SIM): a new approach to nephrotic disease". Biomedical Optics Express. 7 (2): 302–11. doi:10.1364/BOE.7.000302. PMC 4771450. PMID 26977341.
- ^ Liu J, Wang M, Tulman D, Mandava SH, Elfer KN, Gabrielson A, et al. (December 2016). "Nondestructive Diagnosis of Kidney Cancer on 18-gauge Core Needle Renal Biopsy Using Dual-color Fluorescence Structured Illumination Microscopy". Urology. 98: 195–199. doi:10.1016/j.urology.2016.08.036. PMC 5553202. PMID 27597632.
- ^ Westmoreland D, Shaw M, Grimes W, Metcalf DJ, Burden JJ, Gomez K, et al. (April 2016). "Super-resolution microscopy as a potential approach to diagnosis of platelet granule disorders". Journal of Thrombosis and Haemostasis. 14 (4): 839–49. doi:10.1111/jth.13269. PMC 4982064. PMID 26806224.
- ^ Guerra JM (1995). "Super-resolution through diffraction-born evanescent waves". Appl. Phys. Lett. 66 (26): 3555. Bibcode:1995ApPhL..66.3555G. doi:10.1063/1.113814.
- ^ a b U.S. Patent Number 5,774,221, Apparatus and methods for providing phase-controlled evanescent illumination (1996); Number 5,666,197, Apparatus and methods employing phase control and analysis of evanescent for imaging and metrology of subwavelength lateral surface topography (1996), and Number 5,715,059, Dark field, photon tunneling systems and methods (1996)
- ^ a b c Reymann J, Baddeley D, Gunkel M, Lemmer P, Stadter W, Jegou T, et al. (2008). "High-precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy". Chromosome Research. 16 (3): 367–82. doi:10.1007/s10577-008-1238-2. PMID 18461478.
- ^ Heintzmann R, Cremer C (1999). Bigio IJ, Schneckenburger H, Slavik J, Svanberg K, Viallet PM (eds.). "Lateral modulated excitation microscopy: Improvement of resolution by using a diffraction grating". Proc. SPIE. Optical Biopsies and Microscopic Techniques III. 3568: 185–196. Bibcode:1999SPIE.3568..185H. doi:10.1117/12.336833. S2CID 128763403.
- ^ US patent 7,342,717, filed 10 July 1997: Christoph Cremer, Michael Hausmann, Joachim Bradl, Bernhard Schneider Wave field microscope with detection point spread function
- ^ Best G, Amberger R, Baddeley D, Ach T, Dithmar S, Heintzmann R, Cremer C (June 2011). "Structured illumination microscopy of autofluorescent aggregations in human tissue". Micron. 42 (4): 330–5. doi:10.1016/j.micron.2010.06.016. PMID 20926302.
- ^ Hugelier, Siewert; Colosi, P.L.; Lakadamyali, Melike (9 May 2023). "Quantitative Single-Molecule Localization Microscopy". Annual Review of Biophysics. 52 (1): 139–160. doi:10.1146/annurev-biophys-111622-091212. ISSN 1936-122X. PMC 11268362.
- ^ Hell SW, Wichmann J (June 1994). "Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy". Optics Letters. 19 (11): 780–2. Bibcode:1994OptL...19..780H. doi:10.1364/OL.19.000780. PMID 19844443.
- ^ Klar TA, Hell SW (July 1999). "Subdiffraction resolution in far-field fluorescence microscopy". Optics Letters. 24 (14): 954–6. Bibcode:1999OptL...24..954K. doi:10.1364/OL.24.000954. PMID 18073907.
- ^ Kwon J, Lim Y, Jung J, Kim SK (June 2012). "New sub-diffraction-limit microscopy technique: dual-point illumination AND-gate microscopy on nanodiamonds (DIAMOND)". Optics Express. 20 (12): 13347–56. Bibcode:2012OExpr..2013347K. doi:10.1364/OE.20.013347. PMID 22714363.
- ^ Graciani G, Amblard F (December 2019). "Super-resolution provided by the arbitrarily strong superlinearity of the blackbody radiation". Nature Communications. 10 (1): 5761. Bibcode:2019NatCo..10.5761G. doi:10.1038/s41467-019-13780-4. PMC 6917796. PMID 31848354.
- ^ Fernández-Suárez M, Ting AY (December 2008). "Fluorescent probes for super-resolution imaging in living cells". Nature Reviews Molecular Cell Biology. 9 (12): 929–43. doi:10.1038/nrm2531. PMID 19002208. S2CID 2752640.
- ^ Hell SW (May 2007). "Far-field optical nanoscopy". Science. 316 (5828): 1153–8. Bibcode:2007Sci...316.1153H. doi:10.1126/science.1137395. hdl:11858/00-001M-0000-0012-E11C-1. PMID 17525330.
- ^ Huang B, Bates M, Zhuang X (2009). "Super-resolution fluorescence microscopy". Annual Review of Biochemistry. 78: 993–1016. doi:10.1146/annurev.biochem.77.061906.092014. PMC 2835776. PMID 19489737.
- ^ Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW (April 2006). "STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis". Nature. 440 (7086): 935–9. Bibcode:2006Natur.440..935W. doi:10.1038/nature04592. PMID 16612384. S2CID 4326130.
- ^ Patterson GH (October 2009). "Fluorescence microscopy below the diffraction limit". Seminars in Cell & Developmental Biology. 20 (8): 886–93. doi:10.1016/j.semcdb.2009.08.006. PMC 2784032. PMID 19698798.
- ^ Westphal V, Lauterbach MA, Di Nicola A, Hell SW (2007). "Dynamic far-field nanoscopy". New Journal of Physics. 9 (12): 435. Bibcode:2007NJPh....9..435W. doi:10.1088/1367-2630/9/12/435.
- ^ Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW (April 2008). "Video-rate far-field optical nanoscopy dissects synaptic vesicle movement". Science. 320 (5873): 246–9. Bibcode:2008Sci...320..246W. doi:10.1126/science.1154228. PMID 18292304. S2CID 14169050.
- ^ Chmyrov A, Arden-Jacob J, Zilles A, Drexhage KH, Widengren J (November 2008). "Characterization of new fluorescent labels for ultra-high resolution microscopy". Photochemical & Photobiological Sciences. 7 (11): 1378–85. doi:10.1039/B810991P. PMID 18958325. S2CID 31540725.
- ^ Gustafsson MG (September 2005). "Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution". Proceedings of the National Academy of Sciences of the United States of America. 102 (37): 13081–6. Bibcode:2005PNAS..10213081G. doi:10.1073/pnas.0406877102. PMC 1201569. PMID 16141335.
- ^ Guerra JM (1995). "Super-resolution through diffraction-born evanescent waves". Appl. Phys. Lett. 66 (26): 3555–3557. Bibcode:1995ApPhL..66.3555G. doi:10.1063/1.113814.
- ^ von Diezmann A, Shechtman Y, Moerner WE (June 2017). "Three-Dimensional Localization of Single Molecules for Super-Resolution Imaging and Single-Particle Tracking". Chemical Reviews. 117 (11): 7244–7275. doi:10.1021/acs.chemrev.6b00629. PMC 5471132. PMID 28151646.
- ^ Thompson RE, Larson DR, Webb WW (May 2002). "Precise nanometer localization analysis for individual fluorescent probes". Biophysical Journal. 82 (5): 2775–83. Bibcode:2002BpJ....82.2775T. doi:10.1016/S0006-3495(02)75618-X. PMC 1302065. PMID 11964263.
- ^ Mortensen KI, Churchman LS, Spudich JA, Flyvbjerg H (May 2010). "Optimized localization analysis for single-molecule tracking and super-resolution microscopy". Nature Methods. 7 (5): 377–81. doi:10.1038/nmeth.1447. PMC 3127582. PMID 20364147.
- ^ a b Weisenburger S, Boening D, Schomburg B, Giller K, Becker S, Griesinger C, Sandoghdar V (February 2017). "Cryogenic optical localization provides 3D protein structure data with Angstrom resolution". Nature Methods. 14 (2): 141–144. doi:10.1038/nmeth.4141. hdl:11858/00-001M-0000-002C-DE99-3. PMID 28068317. S2CID 4092897.
- ^ a b c Lemmer P, Gunkel M, Baddeley D, Kaufmann R, Urich A, Weiland Y, Reymann J, Müller P, Hausmann M, Cremer C (2008). "SPDM: Light Microscopy with Single Molecule Resolution at the Nanoscale" (PDF). Applied Physics B. 93 (1): 1–12. Bibcode:2008ApPhB..93....1L. doi:10.1007/s00340-008-3152-x. S2CID 13805053.
- ^ Van Oijen AM, Köhler J, Schmidt J, Müller M, Brakenhoff GJ (31 July 1998). "3-Dimensional super-resolution by spectrally selective imaging" (PDF). Chemical Physics Letters. 292 (1–2): 183–187. Bibcode:1998CPL...292..183V. doi:10.1016/S0009-2614(98)00673-3.
- ^ Sätzler B, Cremer E (1 February 1998). "High-precision distance measurements and volume-conserving segmentation of objects near and below the resolution limit in three-dimensional confocal fluorescence microscopy". Journal of Microscopy. 189 (2): 118–136. doi:10.1046/j.1365-2818.1998.00276.x. S2CID 73578516.
- ^ Reymann J, Baddeley D, Gunkel M, Lemmer P, Stadter W, Jegou T, et al. (May 2008). "High-precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy". Chromosome Research. 16 (3): 367–82. doi:10.1007/s10577-008-1238-2. PMID 18461478.
- ^ Cremer C, Kaufmann R, Gunkel M, Pres S, Weiland Y, Müller P, et al. (September 2011). "Superresolution imaging of biological nanostructures by spectral precision distance microscopy". Biotechnology Journal. 6 (9): 1037–51. doi:10.1002/biot.201100031. PMID 21910256. S2CID 21253369.
- ^ Cremer C, Kaufmann R, Gunkel M, Polanski F, Müller P, Dierkes R, Degenhard S, Wege C, Hausmann M, Birk U (July 2014). "Application perspectives of localization microscopy in virology". Histochemistry and Cell Biology. 142 (1): 43–59. doi:10.1007/s00418-014-1203-4. PMID 24614971. S2CID 16930362.
- ^ Wang Q, Dierkes R, Kaufmann R, Cremer C (April 2014). "Quantitative analysis of individual hepatocyte growth factor receptor clusters in influenza A virus infected human epithelial cells using localization microscopy". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1838 (4): 1191–8. doi:10.1016/j.bbamem.2013.12.014. PMID 24374315.
- ^ Gunkel M, Erdel F, Rippe K, Lemmer P, Kaufmann R, Hörmann C, Amberger R, Cremer C (June 2009). "Dual color localization microscopy of cellular nanostructures" (PDF). Biotechnology Journal. 4 (6): 927–38. doi:10.1002/biot.200900005. PMID 19548231. S2CID 18162278.
- ^ Zondervan R, Kulzer F, Kolchenko M, Orrit M (2004). "Photobleaching of Rhodamine 6G in Poly(vinyl alcohol) at the Ensemble and Single-Molecule Levels". J. Phys. Chem. A. 108 (10): 1657–1665. Bibcode:2004JPCA..108.1657Z. doi:10.1021/jp037222e.
- ^ Weisenburger S, Jing B, Renn A, Sandoghdar V (2013). Verma P, Egner A (eds.). "Cryogenic localization of single molecules with angstrom precision". Proc. SPIE. Nanoimaging and Nanospectroscopy. 8815: 88150D. Bibcode:2013SPIE.8815E..0DW. doi:10.1117/12.2025373. S2CID 120610755.
- ^ Schoen I, Ries J, Klotzsch E, Ewers H, Vogel V (September 2011). "Binding-activated localization microscopy of DNA structures" (PDF). Nano Letters. 11 (9): 4008–11. Bibcode:2011NanoL..11.4008S. doi:10.1021/nl2025954. PMID 21838238.
- ^ Szczurek A, Klewes L, Xing J, Gourram A, Birk U, Knecht H, Dobrucki JW, Mai S, Cremer C (2017). "Imaging chromatin nanostructure with binding-activated localization microscopy based on DNA structure fluctuations". Nucleic Acids Research. 45 (8): gkw1301. doi:10.1093/nar/gkw1301. PMC 5416826. PMID 28082388.
- ^ Ries J, Udayar V, Soragni A, Hornemann S, Nilsson KP, Riek R, et al. (July 2013). "Superresolution imaging of amyloid fibrils with binding-activated probes". ACS Chemical Neuroscience. 4 (7): 1057–61. doi:10.1021/cn400091m. PMC 3715833. PMID 23594172.
- ^ Huh H, Lee J, Kim HJ, Hohng S, Kim SK (2017). "Morphological analysis of oligomeric vs. fibrillar forms of α-synuclein aggregates with super-resolution BALM imaging". Chemical Physics Letters. 690: 62–67. Bibcode:2017CPL...690...62H. doi:10.1016/j.cplett.2017.10.034.
- ^ a b Rust MJ, Bates M, Zhuang X (October 2006). "Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM)". Nature Methods. 3 (10): 793–5. doi:10.1038/nmeth929. PMC 2700296. PMID 16896339.
- ^ a b c Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. (September 2006). "Imaging intracellular fluorescent proteins at nanometer resolution". Science. 313 (5793): 1642–5. Bibcode:2006Sci...313.1642B. doi:10.1126/science.1127344. PMID 16902090.
- ^ a b c Hess ST, Girirajan TP, Mason MD (December 2006). "Ultra-high resolution imaging by fluorescence photoactivation localization microscopy". Biophysical Journal. 91 (11): 4258–72. Bibcode:2006BpJ....91.4258H. doi:10.1529/biophysj.106.091116. PMC 1635685. PMID 16980368.
- ^ Zhuang X (2009). "Nano-imaging with Storm". Nature Photonics. 3 (7): 365–367. Bibcode:2009NaPho...3..365Z. doi:10.1038/nphoton.2009.101. PMC 2840648. PMID 20300445.
- ^ Bates M, Blosser TR, Zhuang X (March 2005). "Short-range spectroscopic ruler based on a single-molecule optical switch". Physical Review Letters. 94 (10): 108101. arXiv:q-bio/0502012. Bibcode:2005PhRvL..94j8101B. doi:10.1103/physrevlett.94.108101. PMC 2652517. PMID 15783528.
- ^ Dempsey GT, Bates M, Kowtoniuk WE, Liu DR, Tsien RY, Zhuang X (December 2009). "Photoswitching mechanism of cyanine dyes". Journal of the American Chemical Society. 131 (51): 18192–3. doi:10.1021/ja904588g. PMC 2797371. PMID 19961226.
- ^ a b Bock H, Geisler C, Wurm CA, Von Middendorff C, Jakobs S, Schönle A, et al. (2007). "Two-color far-field fluorescence nanoscopy based on photoswitchable emitters". Applied Physics B. 88 (2): 161–165. Bibcode:2007ApPhB..88..161B. doi:10.1007/s00340-007-2729-0. S2CID 122146697.
- ^ Fölling J, Bossi M, Bock H, Medda R, Wurm CA, Hein B, et al. (November 2008). "Fluorescence nanoscopy by ground-state depletion and single-molecule return". Nature Methods. 5 (11): 943–5. doi:10.1038/nmeth.1257. hdl:11858/00-001M-0000-0012-DA73-1. PMID 18794861. S2CID 205418732.
- ^ Heilemann M, van de Linde S, Schüttpelz M, Kasper R, Seefeldt B, Mukherjee A, et al. (2008). "Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes". Angewandte Chemie. 47 (33): 6172–6. doi:10.1002/anie.200802376. PMID 18646237. S2CID 2743064.
- ^ Heilemann M, van de Linde S, Mukherjee A, Sauer M (2009). "Super-resolution imaging with small organic fluorophores". Angewandte Chemie. 48 (37): 6903–8. doi:10.1002/anie.200902073. PMID 19670280. S2CID 8942815.
- ^ Vogelsang J, Cordes T, Forthmann C, Steinhauer C, Tinnefeld P (May 2009). "Controlling the fluorescence of ordinary oxazine dyes for single-molecule switching and superresolution microscopy". Proceedings of the National Academy of Sciences of the United States of America. 106 (20): 8107–12. Bibcode:2009PNAS..106.8107V. doi:10.1073/pnas.0811875106. PMC 2688868. PMID 19433792.
- ^ Lee HL, Lord SJ, Iwanaga S, Zhan K, Xie H, Williams JC, et al. (November 2010). "Superresolution imaging of targeted proteins in fixed and living cells using photoactivatable organic fluorophores". Journal of the American Chemical Society. 132 (43): 15099–101. doi:10.1021/ja1044192. PMC 2972741. PMID 20936809.
- ^ a b c Bates M, Huang B, Dempsey GT, Zhuang X (September 2007). "Multicolor super-resolution imaging with photo-switchable fluorescent probes". Science. 317 (5845): 1749–53. Bibcode:2007Sci...317.1749B. doi:10.1126/science.1146598. PMC 2633025. PMID 17702910.
- ^ a b c d Huang B, Jones SA, Brandenburg B, Zhuang X (December 2008). "Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution". Nature Methods. 5 (12): 1047–52. doi:10.1038/nmeth.1274. PMC 2596623. PMID 19029906.
- ^ a b Dani A, Huang B, Bergan J, Dulac C, Zhuang X (December 2010). "Superresolution imaging of chemical synapses in the brain". Neuron. 68 (5): 843–56. doi:10.1016/j.neuron.2010.11.021. PMC 3057101. PMID 21144999.
- ^ Testa I, Wurm CA, Medda R, Rothermel E, von Middendorf C, Fölling J, et al. (October 2010). "Multicolor fluorescence nanoscopy in fixed and living cells by exciting conventional fluorophores with a single wavelength". Biophysical Journal. 99 (8): 2686–94. Bibcode:2010BpJ....99.2686T. doi:10.1016/j.bpj.2010.08.012. PMC 2956215. PMID 20959110.
- ^ a b c d Jones SA, Shim SH, He J, Zhuang X (June 2011). "Fast, three-dimensional super-resolution imaging of live cells". Nature Methods. 8 (6): 499–508. doi:10.1038/nmeth.1605. PMC 3137767. PMID 21552254.
- ^ a b Wang W, Li GW, Chen C, Xie XS, Zhuang X (September 2011). "Chromosome organization by a nucleoid-associated protein in live bacteria". Science. 333 (6048): 1445–9. Bibcode:2011Sci...333.1445W. doi:10.1126/science.1204697. PMC 3329943. PMID 21903814.
- ^ a b Huang B, Wang W, Bates M, Zhuang X (February 2008). "Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy". Science. 319 (5864): 810–3. Bibcode:2008Sci...319..810H. doi:10.1126/science.1153529. PMC 2633023. PMID 18174397.
- ^ Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P (September 2010). "Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system". Nature Cell Biology. 12 (9): 902–8. doi:10.1038/ncb2094. PMC 3338250. PMID 20729836.
- ^ Sharonov A, Hochstrasser RM (December 2006). "Wide-field subdiffraction imaging by accumulated binding of diffusing probes". Proceedings of the National Academy of Sciences of the United States of America. 103 (50): 18911–6. Bibcode:2006PNAS..10318911S. doi:10.1073/pnas.0609643104. PMC 1748151. PMID 17142314.
- ^ a b Shah, Shalin; Dubey, Abhishek K.; Reif, John (10 April 2019). "Programming Temporal DNA Barcodes for Single-Molecule Fingerprinting". Nano Letters. 19 (4): 2668–2673. Bibcode:2019NanoL..19.2668S. doi:10.1021/acs.nanolett.9b00590. ISSN 1530-6984. PMID 30896178. S2CID 84841635.
- ^ Shah, Shalin; Dubey, Abhishek K.; Reif, John (17 May 2019). "Improved Optical Multiplexing with Temporal DNA Barcodes". ACS Synthetic Biology. 8 (5): 1100–1111. doi:10.1021/acssynbio.9b00010. PMID 30951289. S2CID 96448257.
- ^ Tinnefeld, Philip; et al. (2015). Far-Field Optical Nanoscopy. Springer. p. 334. ISBN 978-3-662-45547-0. Retrieved 6 July 2020.
- ^ Lew MD, Lee SF, Ptacin JL, Lee MK, Twieg RJ, Shapiro L, Moerner WE (November 2011). "Three-dimensional superresolution colocalization of intracellular protein superstructures and the cell surface in live Caulobacter crescentus". Proceedings of the National Academy of Sciences of the United States of America. 108 (46): E1102-10. doi:10.1073/pnas.1114444108. PMC 3219151. PMID 22031697.
- ^ Pyle JR, Chen J (2 November 2017). "Photobleaching of YOYO-1 in super-resolution single DNA fluorescence imaging". Beilstein Journal of Nanotechnology. 8: 2296–2306. doi:10.3762/bjnano.8.229. PMC 5687005. PMID 29181286.
- ^ Jungmann R, Steinhauer C, Scheible M, Kuzyk A, Tinnefeld P, Simmel FC (November 2010). "Single-molecule kinetics and super-resolution microscopy by fluorescence imaging of transient binding on DNA origami". Nano Letters. 10 (11): 4756–61. Bibcode:2010NanoL..10.4756J. doi:10.1021/nl103427w. PMID 20957983. S2CID 11788360.
- ^ Nieves DJ, Gaus K, Baker MA (December 2018). "DNA-Based Super-Resolution Microscopy: DNA-PAINT". Genes. 9 (12): 621. doi:10.3390/genes9120621. PMC 6315775. PMID 30544986.
- ^ Chen J, Bremauntz A, Kisley L, Shuang B, Landes CF (October 2013). "Super-resolution mbPAINT for optical localization of single-stranded DNA". ACS Applied Materials & Interfaces. 5 (19): 9338–43. doi:10.1021/am403984k. PMC 3934010. PMID 24073628.
- ^ Kisley L, Chen J, Mansur AP, Shuang B, Kourentzi K, Poongavanam MV, et al. (February 2014). "Unified superresolution experiments and stochastic theory provide mechanistic insight into protein ion-exchange adsorptive separations". Proceedings of the National Academy of Sciences of the United States of America. 111 (6): 2075–80. Bibcode:2014PNAS..111.2075K. doi:10.1073/pnas.1318405111. PMC 3926075. PMID 24459184.
- ^ Wang W, Shen H, Shuang B, Hoener BS, Tauzin LJ, Moringo NA, et al. (November 2016). "Super Temporal-Resolved Microscopy (STReM)". The Journal of Physical Chemistry Letters. 7 (22): 4524–4529. doi:10.1021/acs.jpclett.6b02098. PMID 27797527. S2CID 25798776.
- ^ Kaufmann R, Müller P, Hausmann M, Cremer C (June 2011). "Imaging label-free intracellular structures by localisation microscopy". Micron. 42 (4): 348–52. doi:10.1016/j.micron.2010.03.006. PMID 20538472.
- ^ Ayas S, Cinar G, Ozkan AD, Soran Z, Ekiz O, Kocaay D, et al. (2013). "Label-free nanometer-resolution imaging of biological architectures through surface enhanced Raman scattering". Scientific Reports. 3: 2624. Bibcode:2013NatSR...3E2624A. doi:10.1038/srep02624. PMC 3769681. PMID 24022059.
- ^ Heilemann et al., 2008, Angewandte Chemie and van de Linde et al., 2011, Photochem. Photobiol. Sci
- ^ Baddeley D, Jayasinghe ID, Cremer C, Cannell MB, Soeller C (January 2009). "Light-induced dark states of organic fluochromes enable 30 nm resolution imaging in standard media". Biophysical Journal. 96 (2): L22-4. Bibcode:2009BpJ....96L..22B. doi:10.1016/j.bpj.2008.11.002. PMC 2716455. PMID 19167284.
- ^ Fölling J, Bossi M, Bock H, Medda R, Wurm CA, Hein B, et al. (November 2008). "Fluorescence nanoscopy by ground-state depletion and single-molecule return". Nature Methods. 5 (11): 943–5. doi:10.1038/NMETH.1257. hdl:11858/00-001M-0000-0012-DA73-1. PMID 18794861. S2CID 205418732.
- ^ Heilemann M, van de Linde S, Schüttpelz M, Kasper R, Seefeldt B, Mukherjee A, et al. (2008). "Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes". Angewandte Chemie. 47 (33): 6172–6. doi:10.1002/anie.200802376. PMID 18646237. S2CID 2743064.
- ^ Sage D, Kirshner H, Pengo T, Stuurman N, Min J, Manley S, Unser M (August 2015). "Quantitative evaluation of software packages for single-molecule localization microscopy". Nature Methods. 12 (8): 717–24. doi:10.1038/nmeth.3442. PMID 26076424. S2CID 11781779.
- ^ "Single-Molecule Localization Microscopy – Software Benchmarking". Biomedical Imaging Group, Ecole Polytechnique Fédérale de Lausanne (EPFL). 2018. Retrieved 7 July 2020.
- ^ Baddeley D, Cannell MB, Soeller C (February 2010). "Visualization of localization microscopy data". Microscopy and Microanalysis. 16 (1): 64–72. Bibcode:2010MiMic..16...64B. doi:10.1017/S143192760999122X. PMID 20082730. S2CID 10394084.
- ^ Prakash K (17 May 2017). "High-density superresolution microscopy with an incoherent light source and a conventional epifluorescence microscope setup". bioRxiv 10.1101/121061.
- ^ Reinhardt, Susanne C. M.; Masullo, Luciano A.; Baudrexel, Isabelle; Steen, Philipp R.; Kowalewski, Rafal; Eklund, Alexandra S.; Strauss, Sebastian; Unterauer, Eduard M.; Schlichthaerle, Thomas; Strauss, Maximilian T.; Klein, Christian; Jungmann, Ralf (May 2023). "Ångström-resolution fluorescence microscopy". Nature. 617 (7962): 711–716. Bibcode:2023Natur.617..711R. doi:10.1038/s41586-023-05925-9. ISSN 1476-4687. PMC 10208979. PMID 37225882.
- ^ Baddeley D, Batram C, Weiland Y, Cremer C, Birk UJ.: Nanostructure analysis using Spatially Modulated Illumination microscopy. In: Nature Protocols 2007; 2: 2640–2646
- ^ Kaufmann R, Müller P, Hildenbrand G, Hausmann M, Cremer C (2010). "Analysis of Her2/neu membrane protein clusters in different types of breast cancer cells using localization microscopy". Journal of Microscopy. 242 (1): 46–54. doi:10.1111/j.1365-2818.2010.03436.x. PMID 21118230. S2CID 2119158.
- ^ Liss V, Barlag B, Nietschke M, Hensel M (December 2015). "Self-labelling enzymes as universal tags for fluorescence microscopy, super-resolution microscopy and electron microscopy". Scientific Reports. 5: 17740. Bibcode:2015NatSR...517740L. doi:10.1038/srep17740. PMC 4672345. PMID 26643905.
- ^ Ledig C, Theis L, Huszar F, Caballero J, Cunningham A, Acosta A, Aitken A, Tejani A, Totz J (July 2017). "Photo-Realistic Single Image Super-Resolution Using a Generative Adversarial Network". 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Honolulu, HI: IEEE. pp. 105–114. arXiv:1609.04802. doi:10.1109/CVPR.2017.19. ISBN 978-1-5386-0457-1. S2CID 211227.
- ^ Rivenson Y, Göröcs Z, Günaydin H, Zhang Y, Wang H, Ozcan A (20 November 2017). "Deep learning microscopy". Optica. 4 (11): 1437. arXiv:1705.04709. Bibcode:2017Optic...4.1437R. doi:10.1364/OPTICA.4.001437. ISSN 2334-2536. S2CID 3045634.
- ^ Grant-Jacob JA, Mackay BS, Baker JA, Xie Y, Heath DJ, Loxham M, Eason RW, Mills B (18 June 2019). "A neural lens for super-resolution biological imaging". Journal of Physics Communications. 3 (6): 065004. Bibcode:2019JPhCo...3f5004G. doi:10.1088/2399-6528/ab267d. ISSN 2399-6528.
- ^ Wang H, Rivenson Y, Jin Y, Wei Z, Gao R, Günaydın H, et al. (January 2019). "Deep learning enables cross-modality super-resolution in fluorescence microscopy". Nature Methods. 16 (1): 103–110. doi:10.1038/s41592-018-0239-0. PMC 7276094. PMID 30559434.
Further reading
[edit]- Marx V (December 2013) [26 November 2013]. "Is super-resolution microscopy right for you?". Technology Feature. Nature Methods (Paper "Nature Reprint Collection, Technology Features"). 10 (12): 1157–63. doi:10.1038/nmeth.2756. PMID 24296472. S2CID 1004998.
- Cremer C, Masters BR (April 2013). "Resolution enhancement techniques in microscopy". The European Physical Journal H. 38 (3): 281–344. Bibcode:2013EPJH...38..281C. doi:10.1140/epjh/e2012-20060-1.